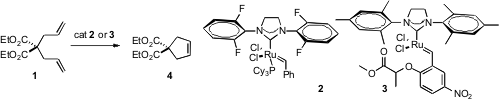
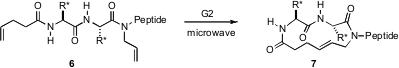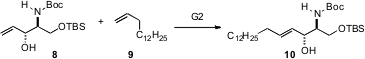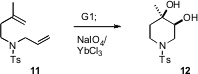Alkene metathesis has become part of the toolkit of organic synthesis. PMID:24182988 Nevertheless, there is room for improvement in catalyst efficiency. 3-Isopropylpyridin-2(1H)-one web Robert H. Grubbs of the California Institute of Technology found (J. Am. Chem. Soc. 2006, 128, 11768.DOI: 10.1021/ja064091x)that the fluorinated complex 1 is several times faster than the widely used G2 ruthenium catalyst. Guy Lavigne of the Laboratoire de Chimie de Coordination, Toulouse, Dieter Arlt of of Ligand Chemie GmbH, Lemgo, Germany and Karol Grela of the Polish Academy of Sciences, Warsaw found (J. Am. Chem. Soc. 2006, 128, 13652.DOI: 10.1021/ja063186w)that 3 is similarly fast, and durable, yielding a TON of 3200 in a ring-closing metathesis.
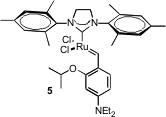
Another approach to high turnover is to immobilize the catalyst. Ned B. Bowden of the University of Iowa has reported (J. Am. Chem. Mal-PEG2-NHS ester supplier Soc. 2006,128, 14434. DOI: 10.1021/ja0642212)the inclusion of G2 in polydimethylsiloxane (PDMS). The polymer excludes water, but organic substrates freely diffuse in and out. With MeOH/water as the reaction medium, the cyclization of 1 proceeded readily, with the catalyst visibly remaining in the solid polymer. Professor Grela and Andreas Kirschning of Leibniz Universität Hannover have described (J. Am. Chem. Soc. 2006, 128, 13261.DOI: 10.1021/ja063561k)a complementary approach, binding 5 and an activating sulfonic acid to glass-polymer Raschig rings.
The special activating effects of microwave irradiation are still actively debated. Paramjit S. Arora of New York University has observed (Org. Lett. 2006, 8, 5825.DOI: 10.1021/ol062443z)that the cyclization of 6 with G2 is sluggish (hours) with oil bath heating, but proceeds rapidly (2 – 5 min) with microwave heating.
Allylic alcohols are particularly useful substrates forcross metathesis. Shigeo Katsumura of Kwansei Kakuin University, Hyogo took advantage of this in developing (Org. Lett. 2006, 8, 5569.DOI: 10.1021/ol062258l)a general route to the sphingolipids and substituted sphingosines. The allylic alcohol 8, readily prepared from L-serine, undergoes smooth cross metathesis with alpha olefins such as 9.
Tandem metathesis-hydrogenation reactions have been described, as have tandem metathesis-alkene migrations, with the Ru species catalyzing both steps. Siegfried Blechert of the Technisches Universität, Berlin (Angew. Chem. Int. Ed. 2006, 45, 1900.DOI: 10.1002/anie.200503552)and Marc L. Snapper of Boston University (Org. Lett. 2006, 8, 4759. DOI: 10.1021/ol061837n)have now reported tandem metathesis-alkene dihydroxylation. For example, cyclization of 11 with catalytic G1 followed by the addition of oxidant delivered the cis diol 12.
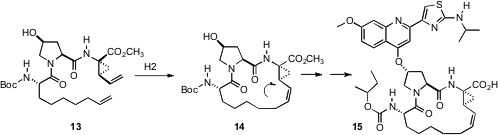
Nathan K. Lee, Vittorio Farina and Kai Donsbach of Boehringer Ingelheim (J. Org. Chem. 2006, 71, 7133,DOI: 10.1021/jo060285jfurther studies by Vittorio Farina and Xudong Wei in J. Org. Chem. 2006,71, 8864. DOI: 10.1021/jo061587o), in the scale-up of the previously-described ( 2005, July 25) metathesis-based synthesis of the hepatitis C protease inhibitor BILN 2061 15, observed epimerization at the indicated center in the product 14. They eventually found that this could be minimized by using the Hoveyda catalyst in the cyclization. A crucial quenching of the catalyst at the end of the metathesis was accomplished by adding mercaptonicotinic acid. With these modifications, this approach has been used to produce > 400 kg of cyclized product.
2005, July 25) metathesis-based synthesis of the hepatitis C protease inhibitor BILN 2061 15, observed epimerization at the indicated center in the product 14. They eventually found that this could be minimized by using the Hoveyda catalyst in the cyclization. A crucial quenching of the catalyst at the end of the metathesis was accomplished by adding mercaptonicotinic acid. With these modifications, this approach has been used to produce > 400 kg of cyclized product.