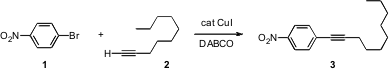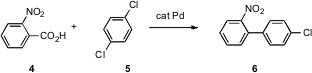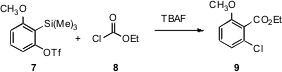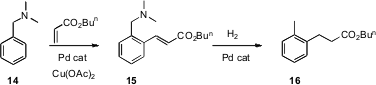There are many ways Pd can be used to catalyze the substitution of an aryl halide. Jin-Heng Li of Hunan Normal University, Changsha, has found (J. Org. Chem. 2007, 72, 2053.DOI: 10.1021/jo0623742)that with the appropriate choice of ligand, Cu can replace Pd in many of these reactions. Ammonium iron(III) citrate custom synthesis Sonogashira (1 + 2 → 3), Suzuki and Heck couplings have been carried out this way. One limitation is that whereas many of the Pd-catalyzed transformations proceed with > 1000 turnovers, the Cu-mediated reactions require 10 mol % catalyst. In related work, Christopher J. Parkinson of CSIR Biosciences, Modderfontein, South Africa, has shown (Tetrahedron Lett. 2007, 48, 3289.DOI: 10.1016/j.tetlet.2007.02.136)that the Cu-mediated coupling of acetoacetate with an aryl halide works well.
An active Pd intermediate for cross-coupling can also be prepared by decarboxylation. This homologation has been studied independently by Lukas J. Goossen of the TU Kaiserslautern (J. Am. Chem. Soc. 2007, 129, 4824. PMID:23291014 DOI: 10.1021/ja068993+)and by Jean-Michel Becht of the Université de Haute-Alsace and Alain Wagner of Novalyst Discovery (Org. 7-Deaza-2′-deoxy-7-iodoadenosine Price Lett. 2007, 9, 1781.DOI: 10.1021/ol070495y). In a related development, Marisa C. Kozlowski of the University of Pennsylvania has established (Org. Lett. 2007, 9, 2441.DOI: 10.1021/ol070749f)a protocol for the reduction decarboxylation of aromatic acids.
Hiroto Yoshida and Atsutaka Kunai of Hiroshima University have developed (Chem. Commun. 2007, 2405.DOI: 10.1039/b701581j)an elegant procedure for the insertion of arynes into acid chlorides, including ethyl chloroformate 8. With the unsymmetrical aryne derived from 7, the addition was directed by the o-methoxy group.
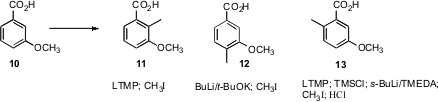
Substitution of a benzene ring can also be effected by directed metalation. Jacques Mortier of the Université du Maine has devised (J. Org. Chem. 2007, 72, 3419.DOI: 10.1021/jo070082a)complementary procedures for the selective metalation and alkylation of m-anisic acid 10. These could be classified as C-H functionalization reactions.
Direct C-H functionalization of benzene derivatives can also be effected using transition metal catalysts. Zhangjie Shi of Peking University has reported (J. Am. Chem. Soc. 2007, 129, 7666.DOI: 10.1021/ja070588a)the Pd-mediated o-homologation of benzyl amines such as 14. Since the benzylic amine is easily hydrogenolyzed, this is also a procedure for selective homologation ortho to the simplest of benzene substituents, a methyl group.
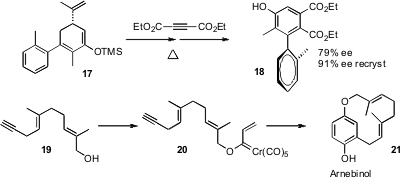
For highly-substituted benzene derivatives, it is sometimes best to build the ring. Two recent contributions are particularly noteworthy. Jiahua Chen, Junmin Quan and Zhen Yang of Peking University found (Org. Lett. 2007, 9, 805. DOI: 10.1021/ol063013b)that dienes such as 17, readily prepared in enantiomerically-pure form from carvone, underwent cycloaddition and fragmentation to form the atropisomeric biphenyls in high ee. Yoko Saikawa and Masaya Nakata of Keio University have shown (Tetrahedron Lett. 2007, 48, 203.DOI: 10.1016/j.tetlet.2006.11.059)that the Dötz cyclization is sufficiently powerful to form even the strained metacyclophane natural product arnebinol 21.