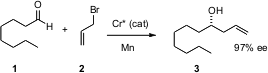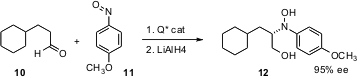Enantioselective allylation is one of the most commonly used methods for constructing secondary alcohols with high enantiocontrol. Hisashi Yamamoto of the University of Chicago has introduced (J. Am. Chem. Soc. 2006,128, 2554. DOI: 10.1021/ja058454p)an improved protocol, a Nozaki-Hiyama coupling with allyl bromide using a chiral Cr catalyst. Mn is the bulk reductant.
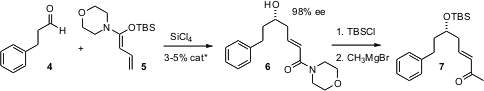
To add a longer chain, Scott E. 408492-27-3 structure 182201-77-0 Purity Denmark of the University of Illinois has developed (J. Am. Chem. Soc. 2006, 128, 1038. PMID:23776646 DOI: 10.1021/ja056747c)a strategy based on SiCl4 activation of ketene silyl hemiaminals such as 5, using 2-5% of a commercially-available organocatalyst. The product6 condenses with CH3MgBr to give the enone 7. More highly substituted ketene silyl hemiaminals also add to aldehydes with high ee.
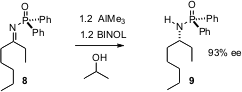
Enantiomerically-pure secondary amines have often been prepared from the corresponding alcohols. Karl A. Scheidt and SonBinh T. Nguyen of Northwestern have devised (Org. Lett. 2006, 8, 1229. DOI: 10.1021/ol060110w)an alternative, a BINOL-mediated imine reduction that proceeds with high ee. Remarkably, the reagent even differentiates between ethyl and pentyl.
Keiji Maruoka of Kyoto University has found (J. Am. Chem. Soc. 2006,128, 6046. DOI: 10.1021/ja0604515)that chiral quaternary ammonium salts effectively catalyze the enantioselective α-amination of an aldehyde. LiAlH4 reduction delivers the amino alcohol 12 in high ee. The advantage of the p-methoxyphenyl protected amine is that the protecting aryl group can be oxidatively removed.
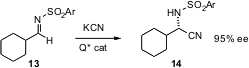
Working in conjunction with Takashi Ooi, also of Kytoto University, Keiji Maruoka had earlier (J. Am. Chem. Soc. 2006, 128, 2548.DOI: 10.1021/ja058066n)shown that the same family of chiral quaternary ammonium catalysts can also be used to form primary amines by enantioselective cyanation of imines such as 13.
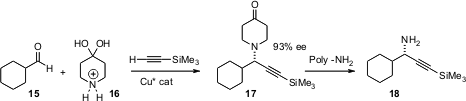
Most methods for the enantioselective construction of primary amines deliver the protected amine. Erick M. Carreira of ETH Zurich has devised (Org. Lett. 2006, 8, 2437.DOI: 10.1021/ol060876w)an approach to propargylic amines that leads to the unprotected primary amine 18. The key to this approach is the use of a polymer-bound primary amine to efficiently transfer divinyl ketone from the initial adduct 17.
The examples above are of amines substituted with secondary centers. The enantioselective construction of amines substituted with quaternary centers is even more challenging. Justin Du Bois of Stanford University has developed (Org. Lett. 2006, 8, 1073.DOI: 10.1021/ol052920y)a general solution to this problem, Rh-mediated insertion into a defined ternary center by an in situ generated Rh nitrene. This paper focuses on guanidines such as 19 and ureas. The Du Bois group had earlier reported Rh-mediated intramolecular C-H amination by carbamates and by sulfamates.