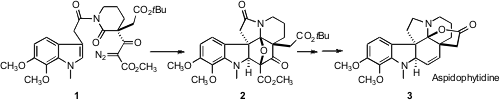
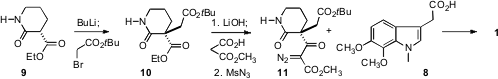Albert Padwa of Emory University has developed (Org. PMID:23664186 Fmoc-Ala-OH supplier Lett. 2006,
8, 3275.
DOI: 10.1021/ol061137i)
a productive approach to fused indole alkaloids such as
aspidophytine (3), based on the
dipolar cycloaddition of the ylide
derived by exposure of a diazo ketones such as 1 to a Rh(II) carboxylate
catalyst.
The preparation of 1 started with the aniline 4. Ortho
iodination followed by N-alkylation with 5 delivered the unsaturated
ester 6. Price of (S)-4-(1-Aminoethyl)phenol hydrobromide Heck cyclization no doubt intially left the alkene still
conjugated with the ester, but traces of acid or base would be expected to
easily isomerize this to establish the aromaticity of the five-membered indole
ring. N-methylation followed by saponification then gave 8.
The preparation of 1 continued with the alkylation of 9.
Hydrolysis of 10 followed by homologation and subsequent diazo transfer
gave 11, which was coupled with 8 to give 1. The quaternary
center established in the alkylation of 9 was carried through the
synthesis, so if enantiomerically-pure material were desired, an
enantioselective route to 10 would have to be devised.
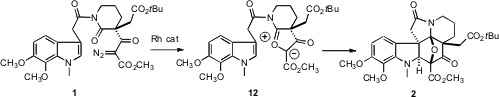
The push-pull dipole 12 was constructed by exposure of 1 to Rh2(OAc)4.
Loss of N2 gave the Rh carbene, which complexed with the nucleophilic
amide carbonyl. The dipole 12 was not isolated, but reacted in situ
with the tethered indole to give the hexacyclic adduct 2. Note that two
diastereomers of 2 could have been formed, but only 2, with the
bulky t-butyl ester exo, was observed.
The dipolar cycloaddition established the requisite stereochemical
relationship between the three contiguous quaternary stereogenic centers of 1.
It remained to adjust the functional groups around the newly-formed
carbocyclic
ring. Exposure to BF3.OEt2 led to ring opening, followed
by trapping of the intermediate carbocation with the t-butyl ester to
give the lactone 13, with concommitant loss of isobutylene. Hydrolysis
and decarboxylation gave the alcohol 14, the acetate of which was removed
by reduction with SmI2. The derived enol triflate 15 was
reduced to the alkene, which was deoxygenated by way of the thiolactam 16.
The intramolecular dipolar cycloaddition exemplified by the conversion of
12 to 2 is a specific representative of a general and powerful
approach to indole alkaloids, based on cycloaddition of an intermediate indole
to a dipole or a diene. For more recent work along these lines by Professor
Padwa, see Org. Lett. 2007, 9, 279,
DOI: 10.1021/ol062728b
and Tetrahedron 2007, 63, 5962,
DOI: 10.1016/j.tet.2007.01.064.