The report last year by scientists at Merck of an antibiotic, platensimycin3, with a novel mechanism of action has led to much effort toward the total synthesis of this degraded diterpene. K. C. PMID:24268253 MC-Val-Cit-PAB manufacturer Nicolaou of Scripps, La Jolla has now (Angew. Chem. Int. Ed. 2006, 45, 7086. DOI: 10.1002/anie.200603892)reported the first preparation of 3. The key step in the synthesis is the elegantly concise cyclization of 1 to 2. 3-Fluoro-2-methyl-6-nitropyridine In stock
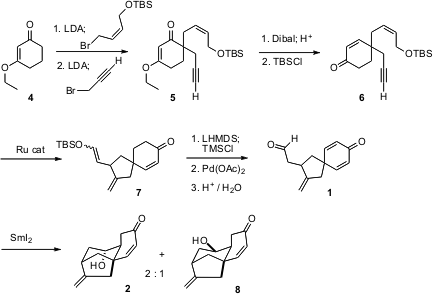
The preparation of 1 started with iterative Stork-Danheiser alkylation of 4 to give 5. Reduction followed by hydrolysis unraveled the enol ether to give the enone, which was re-silylated to give 6. Ru-catalyzed intramolecular ene cyclization of 6 gave the enol ether 7, which was selectively oxidized to 1. Reductive cyclization of 1 gave 2 as a 2:1 mixture with its diastereomer8.
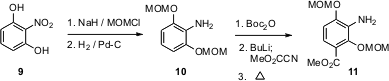
The aniline of platensimycin was prepared from 2-nitroresorcinol (9). MOM ether formation followed by reduction gave 10. Metalation of the protected amine followed by acylation with Mander’s reagent and thermolysis gave 11.
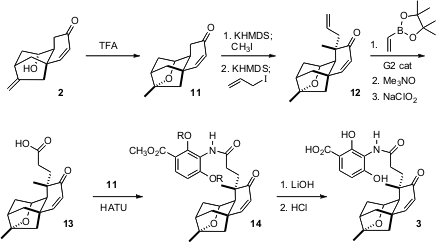
The cyclization of 2 to 11 proceeded smoothly, as did the alkylation of 11 to 12. The diastereoselectivity of the alkylation followed from the conformational bias of the ring system. The conversion of the terminal alkene to the acid 13 initially was troublesome, but a solution was found in metathesis with vinyl boronate followed by oxidation. Coupling with 11 followed by deprotection then gave 3.
The absolute configuration of the tricyclic core of 3 was set in the intramolecular ene cyclization of 6 to 7. There is the possibility that with an enantiomerically-pure catalyst, the product 7 and so 3 could be prepared in high enantiomeric excess.
Total syntheses of platensimycin 3 are underway in several other research groups. The diversity of the approaches being explored will enrich organic synthesis.