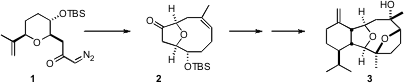Vigulariol (3), isolated from the octocoral Vigularia juncea (Pallas), is active against human lung adenocarcinoma cells (IC50 = 18 nM). A key step in the synthesis of 3 reported (Angew. Chem. PMID:31085260 Int. Ed. 2007, 46, 437.DOI: 10.1002/anie.200603880)by J. Price of 112776-84-8 Stephen Clark of the University of Glasgow was the dipolar rearrangement of1 to 2.
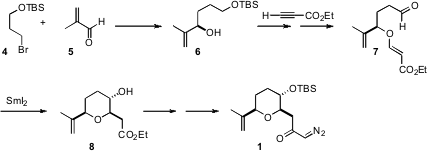
The plan for the diastereoselective construction of 1 was based on the known reductive cyclization of aldehydes such as 7. To this end, the alcohol 6 was prepared and condensed with ethyl propiolate. 56946-65-7 uses As anticipated, the SmI2-mediated cyclization of 7 proceeded to give 8 with high diasterocontrol. The diazo ketone 1 was prepared by reaction of the mixed anhydride (isobutyl chloroformate) of the acid with diazomethane.
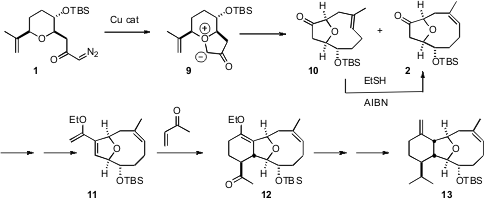
It was anticipated that Cu-catalyzed carbene formation would lead the 1,3-dipole 9, that would then undergo sigmatropic rearrangement to 2. In the event, the rearrangement was remarkably efficient, delivering the ten-membered ring carbocycles 10 and the desired 2 in a 1 : 5 ratio. Exposure of the less stable geometric isomer 10 to catalytic thiyl radical effected equilibration to 2.
Although one might think of medium rings such as that of 2 as being floppy, in fact 2 has a single preferred conformation, and diastereocontrol for the rest of the synthesis took advantage of that preferred conformation. The diene 11 derived from 2 has one open face. Addition of methyl vinyl ketone to that outside face gave a 1 : 2 mixture of the diastereomeric endo and exo cycloadducts. The mixture was equilibrated to the more stable exo adduct 12. A four-step sequence then converted 12 into 13.
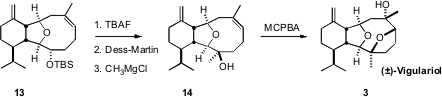
The completion of the synthesis again depended on the inside-outside bias of the medium ring. Addition of methyl magnesium chloride to the outside face of the ketone derived from 13 delivered 14 with high diastereocontrol. Epoxidation of 14, again from the outside face, led directly to vigulariol 3. The intermediate in the MCPBA reaction is the protonated epoxide, and it may be that the tertiary alcohol poised directly on the opposite face of the alkene opened that activated intermediate directly.
This synthesis of vigulariol (3) is remarkably concise, with four rings and eight stereogenic centers being assembled in just twenty steps. All of the stereogenic centers are derived from the secondary alcohol 6. Use of the known enantiomerically-pure 6 would have delivered vigulariol (3) in enantiomerically-pure form. This synthesis design once again illustrates the power of conformational analysis of medium rings.