Jin-Quan Yu of Cambridge University reports (Org. PMID:35567400 Lett. 2003,
5, 4665.
DOI: 10.1021/ol0358509)
that Pd nanoparticles catalyze the hydrogenolysis of benzylic epoxides.
The reaction proceeds with inversion of absolute configuration (1 -> 2).
Laurel Schafer of the University of British Columbia reports (Org. Lett.
2003, 5, 4733.
DOI: 10.1021/ol0359214)
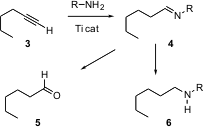
that terminal alkynes undergo smooth hydroamination with a Ti catalyst. Methyl dec-9-enoate Purity 84793-07-7 custom synthesis
The intermediate imine
4 can be hydrolyzed to the aldehyde 5 or reduced directly to the
amine
6. The alkyne to aldehyde conversion has previously been carried out by
hydroboration/oxidation (J. Org. Chem. 1996, 61, 3224.
DOI: 10.1021/jo960386p),
hydrosilylation/oxidation (Tetrahedron Lett.
1984, 25, 321.
DOI: 10.1016/S0040-4039(00)99873-3),
or Ru catalysis (J. Am. Chem. Soc. 2001, 123, 11917.
DOI: 10.1021/ja0119292).
There was no previous general procedure for the anti-Markownikov
conversion of a terminal alkyne to the amine.
The construction of enantiomerically-pure
carbocycles is a general problem in
organic synthesis. Dirk Trauner (UC Berkeley) reports (Org. Lett.
2003, 5, 4113.
DOI: 10.1021/ol035559t)
an elegant intramolecular Heck cyclization. The alcohol
7 is readily prepared in enantiomerically-pure form. Conditions can be
varied so that either
8 or 9 is the dominant product from the cyclization.