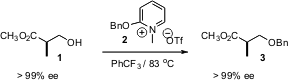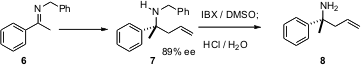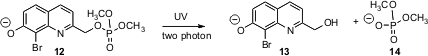Alcohols are the most common functional group protected, and benzyl is the most commonly used alcohol protecting group. Gregory B. Dudley of Florida State University has developed (J. Org. Chem. Buy3-Methoxybenzensulfonyl chloride 2006, 71, 3923.DOI: 10.1021/jo0602773)the reagent2 for benzylation of alcohols under strictly neutral conditions. The environmentally-friendly benzotrifluoride is the solvent of choice for the reaction.
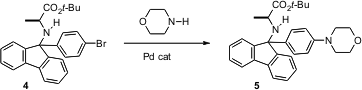
The 9-phenyl-9-fluorenyl group offers many advantages for amine protection. It is stable to mild acid conditions, and removable either with strong acid, hydrogenolysis or reduction. William D. PMID:23695992 Lubell of the Université de Montréal has reported (J. Org. Chem. 2006, 71, 848.DOI: 10.1021/jo0521910)that the 9-(4-bromophenyl)-9-fluorenyl group, illustrated by 4, offers complementary advantages. Stable to acid, it remains in place while Boc groups and t-butyl esters are removed. Pd-mediated coupling with morpholine converts it into 5, which is selectively removed with mild acid in the presence of the Boc, t-butyl ester and 9-phenyl-9-fluorenyl groups. 1,3-Diisopropylimidazolium chloride Price
Many procedures have been developed for enantioselective transformations of aryl and benzyl imines. The problem, then, is removal of the N protecting group. Motomu Kanai and Masakatsu Shibasaki of the University of Tokyo have found (J. Am. Chem. Soc. 2006, 128, 7687.DOI: 10.1021/ja061510h)that the IBX oxidation reported by K. C. Nicolaou is particulary efficient forremoving benzyl protecting groups from amines.
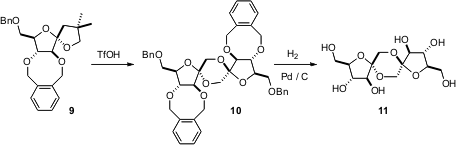
Protecting groups can be used to direct the shape and reactivity of the protected molecule(s). Dimerization of fructose leads to a mixture of the 13 diastereomers of difructose dianhydride, of which 11 is representative. Carmen Ortiz Mellet of the Universidad de Sevilla and José M. García Fernández of CSIC, Sevilla have now shown (Chem. Commun. 2006, 2610.DOI: 10.1039/b604718a)that dimerization of theo-xylylene-protected fructose 9 leads exclusively to 10. Catalytic hydrogenation then delivers 11.
Current advances in biology require that particular areas of a cell be specifically addressed. Two-photon excitation, with the intersecting laser beams coming from different directions, can effect the required three-dimensional control. The challenge has been to develop protecting groups that will release functionality under two-photon excitation. Timothy M. Dore of the University of Georgia has established (J. Am. Chem. Soc. 2006, 128, 4267.DOI: 10.1021/ja0555320)that BHQ (12) can be used to release phosphate (illustrated), carboxylates and alcohols under such two-photon excitation.
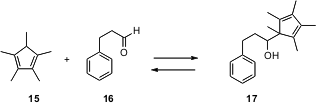
Pentamethylcyclopentadiene (15) was developed as a ligand for transition metals. Now, Hideki Yorimitsu and Koichiro Oshima of Kyoto University have shown (Tetrahedron Lett. 2006, 47, 163.DOI: 10.1016/j.tet.2006.01.097)that15 can be used as a protecting group for aldehydes and ketones. The anion of 15 adds smoothly, to give 17. The addition is reversed by warming with a trace of DDQ.