The aza-Baylis-Hillman (aza-BH) reaction is a nucleophile-catalysed coupling of an imine with an electron-deficient olefin which provides direct access to highly functionalized chiral amines from readily available achiral starting materials. Moreover, the Baylis-Hillman reaction embodies the two very important requirements for good reaction design: atom economy and the generation of functional groups. This is a relatively undeveloped reaction, and the present Highlight focuses on recent developments achieved from 2003 to present (for a comprehensive review on the BH reaction see Chem. Methyl 2-amino-3-hydroxybenzoate Data Sheet Rev. 2003, 103, 811. DOI: 10.1021/cr010043d).
1. Mechanism
No detailed mechanistic information on the aza-BH reaction is available; however, the following general mechanism is currently accepted (Scheme 1).
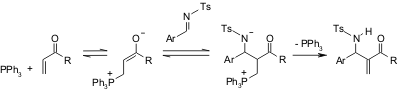
Scheme 1. Proposed mechanism for the aza-BH reaction. H-Lys(Aloc)-OH custom synthesis
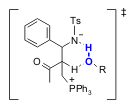
Leitner and co-workers (J. Am. Chem. Soc. 2005, 127, 16762. DOI: 10.1021/ja0550024)recently reported conclusive evidence for the mechanistic involvement of bifunctional activation. This study indicated that the aza-BH reaction involves rate-limiting proton transfer in the absence of added protic species, but exhibits no autocatalysis (Scheme 2). Since the factors responsible for enantiocontrol are not fully understood, these results could help improve future catalyst design.
Scheme 2. Transition state for the Brønsted Acid-assisted proton transfer in the aza-BH.
2. PMID:24914310 Quinuclidine-based catalysts
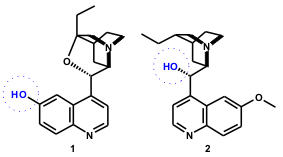
A one-pot, three-component asymmetric aza-Baylis–Hillman reaction was developed by Adolfsson and Balan (Tetrahedron Lett. 2003, 44, 2521. DOI: 10.1016/S0040-4039(03)00289-2)using the chiral quinuclidine-based amine catalysts 1 and 2. With aromatic aldehydes 3, p-toluenesulphonamide (4), and methyl acrylate (5a, 78-95 % yield, 49-74% ee) ort-butyl acrylate (5b, 12% yield, 52% ee) in the presence of 1, an (R)-selectivity was observed. The use of catalyst 2 was found to reverse the selectivity of the reaction (Scheme 3).
Scheme 3. Enantioselective aza-BH reaction using quinuclidine-based catalysts.
3. Phosphine-based catalysts
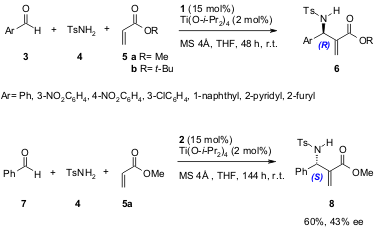
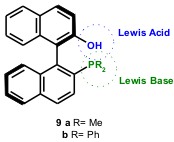
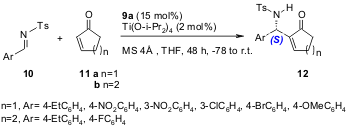
Shi and co-workers (Tetrahedron: Asymmetry, 2005, 16, 1385. DOI: 10.1016/j.tetasy.2005.02.018)have studied extensively the enantioselective aza-BH using the simple chiral phosphine Lewis base catalyst 9a, and recently reported the first example of a catalytic, asymmetric aza-BH using this catalyst. They used N-sulphonated imines 10 and α,β-unsaturated cyclic ketones (9a, 70-93 % yield, 32-55 % ee, 12h; 9b, 66-99 % yield, 14-23 % ee, 24h) as Michael acceptors under mild conditions (Scheme 4).
Scheme 4. Enantioselective aza-BH reaction using the chiral phosphine-based catalyst 9a.
In another recent report (J. Am. Chem. Soc. 2005, 127, 3790. DOI: 10.1021/ja0447255), Shi and co-workers also found that better ee’s could be achieved (for 16) by lowering the reaction temperature (to -30 or -20 °C) and that the use of MS 4Å increased the yield of the reaction using catalyst 9b (Scheme 5).
Scheme 5. Enantioselective aza-BH reaction using the chiral phosphine-based catalyst 9b.
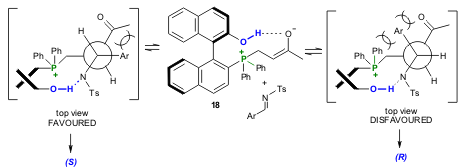
On the basis of NMR studies, the authors believe that 9b acts as a bifunctional chiral ligand where the phosphine atom acts as a Lewis base and the phenolic OH acts as a Lewis acid. The key factor is the intramolecular hydrogen bonding between the phenolic OH and the oxygen atom of carbonyl group to stabilize the key enolate intermediate 18 formed in situ. In addition, the intramolecular hydrogen bonding between the phenolic OH and the nitrogen anion stabilized by the sulphonyl group can facilitate a relatively stable or rigid transition state for achieving high enantioselectivity in the aza-BH reaction (Scheme 6).
Scheme 6. Transition states involved in the enantioselective aza-BH using chiral phosphine catalyst 9b.
4. Thiourea-based catalysts
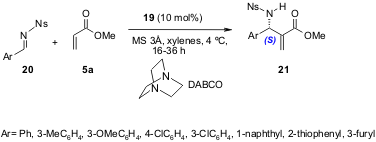
Jacobsen and Raheem (Adv. Synth. Catal. 2005, 347, 1701. DOI: 10.1002/adsc.200505230)developed a highly enantioselective (87-99% ee) catalytic aza-BH reaction of nosylimines 20 with5a, although with very moderate yields (25-49%). The nosyl N-protecting group was found to be the only one that gave high enantioselectivity (Scheme 7).
Scheme 7. Enantioselective aza-BH reaction using the chiral thiourea-based catalyst 19.