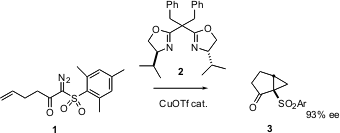
Intramolecular carbene insertion (e.g. 1 -> 3) has long been a useful method for ring construction. Masahisa Nakada of Waseda University in Tokyo now reports (J. Am. Chem. Soc. Buy5-Methoxyquinazolin-4(3H)-one 2003, 125, 2860.DOI: 10.1021/ja029534l)that with the addition of the ligand 2 this process can be made highly enantioselective. PMID:24513027 As the starting diazo ketone 1 is easily prepared by diazo transfer to the sulfonyl ketone, this should allow facile entry to enantioenriched cyclopentanones and cyclohexanones. Price of 2-(4-Nitrophenyl)-2-oxoacetic acid
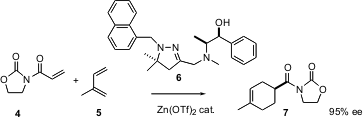
An even more common method for carbacyclic ring construction is the Diels-Alder reaction. Mukund Sibi of North Dakota State University reports (J. Am. Chem. Soc. 2003, 125, 9306.DOI: 10.1021/ja035979d)that the flexible ligand 5 works particularly well in mediating the enantioselective addition of 4 to5, to give 7.
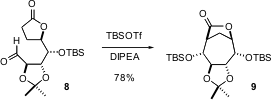
Another way to approach the enantioselective construction of carbocycles is to start with a readily-available carbohydrate. Gloria Rassu of the Insituto di Chimica Biomolecolare del CNR, Sassari, and Giovanni Casiraghi of the Università di Parma report (J. Org. Chem. 2003, 68, 5881.DOI: 10.1021/jo0344539)that the lactone 8 undergoes smooth aldol condensation to give the highly-substituted, and enantiomerically-pure lactone 9. The cyclization works equally well with the lactam in place of the lactone. Eight-membered rings can also be efficiently prepared using this approach.
Intramolecular alkylation, although it is enticing, has not been developed as a method for cyclohexanone construction. Joseph P.A. Harrity of the University of Sheffield reports (J. Org. Chem. 2003, 68, 4392.DOI: 10.1021/jo0300587)that TiCl4 smoothly transforms the enol ether 10, prepared from the corresponding alkynyl phosphonium salt, into the 2-aryl cyclohexanone 11. Alkynyl ethers such as 10 are readily prepared in enantiomerically-enriched form. Would the enantiomeric excess be maintained on cyclization?