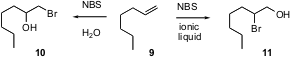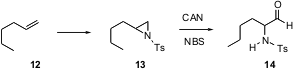Alkenes are among the least expensive of organic functional groups. They are easily incorporated into a growing molecule, and are stable to many of the reagents that transform other functionality. Conversely, it is possible to activate alkenes in the presence of other organic functional groups.
The power of this approach is illustrated by the Ru-catalyzed transborylation of alkenes reported (Chem. Comm. 4-Bromo-3,5-dimethylphenylboronic acid Formula 2005, 663. DOI: 10.1039/b515283f)by Bogdan Marciniec of Adam Mickiewicz University, Poznan. This is not a metathesis reaction, but a transfer of the boryl group from one alkene to the other. ((2-Iodoethoxy)methyl)benzene Formula The products such as 3 are useful building blocks for further coupling. PMID:25429455
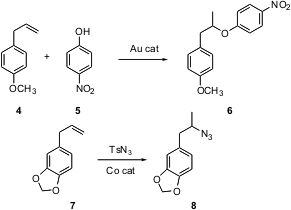
Although hydroboration is well developed as a protocol for the anti-Markovnikov hydration of an alkene, there has not been a comparable environmentally friendly method for Markovnikov functionalization. Now, two such methods have appeared. Chuan He of the University of Chicago has found (J. Am. Chem. Soc. 2005, 127, 6966. DOI: 10.1021/ja050392f)a Au catalyst that mediates the addition of phenols and of carboxylic acids to alkenes. In a related development, Erick M. Carreira of ETH, Zurich has developed (J. Am. Chem. Soc. 2005, 127, 8294. DOI: 10.1021/ja052164r)a Co catalyst that mediates the transformation of an alkene such as 7 to the secondary azide 8.
The bromohydroxylation of a terminal alkene such as 9 usually proceeds to give the primary bromide 10. J.S. Yadav of the Indian Institute of Chemical Technology, Hyderabad has now observed (Tetrahedron Lett. 2005, 46, 3569. DOI: 10.1016/j.tetlet.2005.03.108)that in an ionic liquid, halohydrin formation proceeds with the opposite regioselectivity, leading to 11 as the dominant product.
Aziridines such as 13, both racemic and enantiomerically-enriched, are readily prepared from the corresponding alkene. K. Rama Rao of the Indian Institute of Chemical Technology, Hyderabad, has found (Tetrahedron Lett. 2005, 46, 4111. DOI: 10.1016/j.tetlet.2005.04.008)that a combination of N-bromosuccinimide and ceric ammonium nitrate in MeCN/H2O converts the aziridine 13 to the protected α-amino aldehyde 14. A key question, as yet unanswered, is whether the enantiomeric excess of 13 would be maintained through the oxidation.
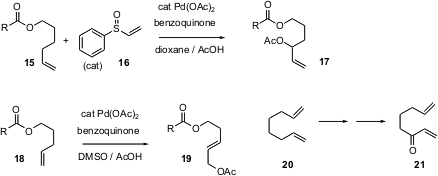
Monosubstituted alkenes such as 15 are among the least expensive of organic functional groups. M. Christina White, now at the University of Illinois, has been developing conditions for specific Pd-catalyzed allylic oxidation of such alkenes. Two products are possible, the branched isomer such as 17 and the linear isomer 19. Professor White has found that in the presence of a catalytic amount of the sulfoxide 16 (J. Am. Chem. Soc. 2005, 127, 6970.DOI: 10.1021/ja0500198)the branched isomer is dominant, while in the presence of DMSO (Org. Lett. 2006, 7, 223. DOI: 10.1021/ol047800p)the product is linear. Internal alkenes are not affected. Among many other applications, one can imagine using such an oxidation to convert the inexpensive butadiene dimer 20 into the valuable bis-annulation synthon 21.