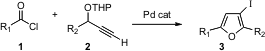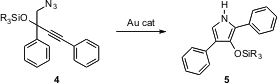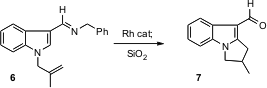Thomas J. J. Müller of the Universität Heidelberg has reported (Chem. Methyltrioxorhenium(VII) structure 5-(Thiazol-5-yl)nicotinic acid web Commun. 2005, 2581.DOI: 10.1039/b502324f)a powerful method for the assembly of highly substituted furans, Pd-mediated condensation of an acid chloride 1 with a propargylic ether 2. Depending on the halide used in the work-up, either chloro or iodofurans are prepared. The reaction works well for R2 = H, opening access to rare 2,4-disubstituted furans.
F. Dean Toste of the University of California, Berkeley, has found (J. Am. Chem. Soc. 2005, 127, 11260.DOI: 10.1021/ja053804t)that exposure of an alkynyl azide such as 4 to an Au catalyst leads to intramolecular Schmidt reaction, to deliver the substituted pyrrole. In the case of 4, the rearrangement proceeds with concomitant migration of the siloxy group, to give 5. PMID:24670464 Alkyl-substituted alkynyl azides and alkynyl azides without the silyloxy groups also rearrange efficiently.
Robert G. Bergman and Jonathan A. Ellman of the University of California, Berkeley, have developed (J. Org. Chem. 2005, 70, 6775.DOI: 10.1021/jo050757e)a C-H activation-based method for the intramolecular alkylation of an aromatic ring. Applied to the indole 6, this leads to the tricyclic indole 7. The procedure works well with pyrroles also, as well as with benzene derivatives.
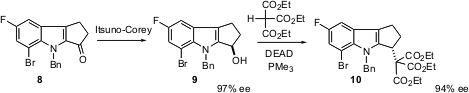
Michael C. Hillier of the Merck Process group in Rahway, NJ was faced (J. Org. Chem. 2005, 70, 8385.DOI: 10.1021/jo051146p)with the challenge of preparing the indole 10 in enantiomerically-pure form. Itsuno-Corey reduction of the ketone 8 worked well, but subsequent Mitsunobu coupling to give 10 led to substantial racemization. The authors eventually found that use of PMe3 allowed nearly perfect inversion in the conversion of 9 to 10.
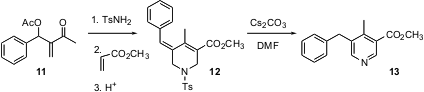
Highly substituted pyridines are often most easily prepared by total synthesis. Jae Nyoung Kim of Chonnam National University, Gwangju has developed (Tetrahedron Lett. 2005, 46, 8799.DOI: 10.1016/j.tetlet.2005.10.034)a new route to pyridines such as 13, based on the addition of tosyl amide to Baylis-Hillman adducts such as 11.
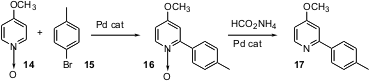
Keith Fagnou of the University of Ottawa has found (J. Am. Chem. Soc. 2005, 127, 18020.DOI: 10.1021/ja056800x)that transition metal-mediated C-H functionalization also works efficiently with pyridine N-oxides. An aryl halide such as 15 couples directly with14 under Pd catalysis to give 16. The product N-oxide is easily reduced to the pyridine 17.
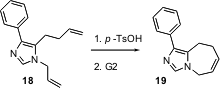
The Grubbs second generation alkene metathesis catalyst (“G2”) is compatible with many organic functional groups. It cannot be used, however, with basic amines. As illustrated by the efficient cyclization of 18 to 19 reported (Org. Lett. 2005, 7, 3183.DOI: 10.1021/ol050852+)by Vijaya Gracias of Abbott Laboratories, Abbott Park, IL, a simple solution to this problems is to pre-treat the amine with a stoichiometric amount of acid.