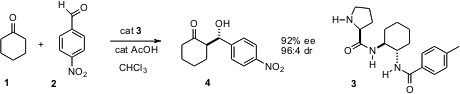
Enantiomerically-pure carbocycles can be prepared either de novo, or by desymmetrization of prochiral rings. A classic illustration of the latter approach is the condensation of cyclohexanone (1) with p-nitrobenzaldehyde (2). Wen-Jing Xiao of Central China Normal University in Wuhan has reported (Org. Lett. 6-Bromopyrazin-2-amine In stock 2005, 7, 4543.DOI: 10.1021/ol0520323)that the L-prolinamide 3 is a particularly effective organocatalyst for this transformation.
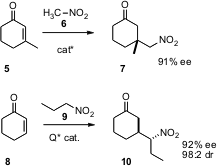
Cyclohexenones such as 5 and 8 are also prochiral. Amino-PEG3-C2-Amine Price Steven V. PMID:28322188 Ley of the University of Cambridge (Chem. Commun. 2005, 5346.DOI: 10.1039/b511441a)and Keiji Maruoka of Kyoto University (Org. Lett. 2005, 7, 5143.DOI: 10.1021/ol0517170)independently developed organocatalysts that effect enantioselective conjugate addition of nitroalkanes. Nitromethane and primary and secondary nitroalkanes participated efficiently in the addition. Addition to acyclic enones also proceeded with high ee. Professor Ley has shown that quaternary centers can be formed with high ee, and Professor Maruoka has shown that the sidechain stereocenter is also controlled. Note that the primary nitro group of adducts such as 7 is readily converted into the nitrile or the carboxylic acid.
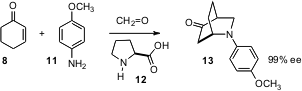
New applications are still being found for proline (12), one of first substances shown to be an enantioselective organocatalyst. Armando Córdova of Stockholm University has found (Angew. Chem. Int. Ed. 2005, 44, 4877. DOI: 10.1002/anie.200500811)that three-component coupling of cyclohexenone (8), formaldehyde, and an aromatic amine 11 catalyzed by proline delivered the bicyclic amine 13 in high ee.
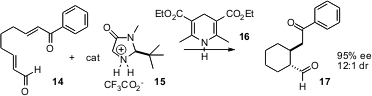
Enantioselective ring construction can also be effected by organocatalysts. Benjamin List of the Max-Planck-Institut, Mülheim has devised (J. Am. Chem. Soc. 2005, 127, 15036.DOI: 10.1021/ja055735o)a combination of organocatalyst 15 and organic reductant 16. The transient iminum salt formed with the aldehyde 14 is reduced to a chiral enamine, that adds in an intramolecular fashion to the acceptor enone, to give17.
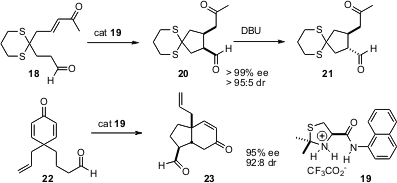
Yujiro Hayashi of the Tokyo University of Science has shown (J. Am. Chem. Soc. 2005, 127, 16028.DOI: 10.1021/ja055740s)that 19 is an excellent catalyst for enantioselective intramolecularMichael addition. The conditions are mild enough that the kinetic cis product 20 is formed exclusively. Exposure to DBU converts 20 to the trans diastereomer 21, without loss of ee. The enantioselective intramolecular Michael addition can also be used to construct bicyclic systems such as 23. As expected for intramolecular addition, the reaction proceeds to give exclusively the cis ring fusion.