Aryl amines are common constituents of pharmaceuticals, so there has been a great deal of interest in their preparation, e.g by Pd- or Cu-mediated amination of aryl halides. Multi-step procedures for monoalkylation of amino arenes have also been developed. Richard A. Hudson of the University of Toledo, OH, has put forward (Org. Lett. 2005, 7, 471.DOI: 10.1021/ol047580f)a complementary approach, the mono N-alkylation of nitrobenzenes such as 1 by reduction in the presence of a nitrile 2. Another way of seeing this transformation is as the mono-N-arylation of the primary amine derived by reduction of the nitrile 2, using the nitroaromatic 1 as the source of the arene. The nitroaromatic 1 can be replaced in these transformations by the corresponding primary aniline.
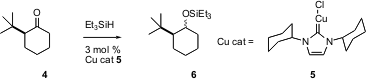
Ketones such as 4 can be converted to the silyl ether 6 by reduction followed by protection. PMID:24101108 7-Chloro-L-tryptophan Order Previous catalysts for the alternative one step procedure, direct hydrosilylation, have often failed with more hindered ketones. 7-Bromo-2-methyloxazolo[4,5-c]pyridine Data Sheet Steven P. Nolan of the University of New Orleans has now (J. Org. Chem. 2005, 70, 4784.DOI: 10.1021/jo050397v)developed a robust Cu carbene complex 5 that effectively catalyzes the reductive silylation of even very hindered ketones.
Alcohol deoxygenation is usually carried out by free radical reduction. Alternatively, hydride reagents can be used to reduce some sulfonates. Gabriel Radivoy of the Universidad Nacional del Sur, Argentina, and Miguel Yus of the Universidad de Alicante have found (Tetrahedron 2005, 61, 3859. DOI: 10.1016/j.tet.2005.01.078)that low-valent copper smoothly mediates the reduction of primary, secondary, and tertiary mesylates including 7. This reagent combination will also reduce enol sulfonates such as 10, leaving the alkene or diene intact.
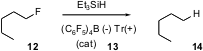
Carbon-fluorine bonds are resistant to direct reduction. Oleg V. Ozerov of Brandeis University has taken (J. Am. Chem. Soc. 2005, 127, 2852. DOI: 10.1021/ja0426138)an alternative approach to this problem, setting up a catalytic cycle that is initiated by trityl cation (13). Hydride abstraction fromEt3SiH by the trityl cation gives Et3Si(+), that then extracts F from 12. The transient or incipient carbocation so produced abstracts a hydride from Et3SiH, regenerating Et3Si(+) and so continuing the catalytic cycle. Aryl C-F and benzylic C-F bonds are also reduced smoothly.
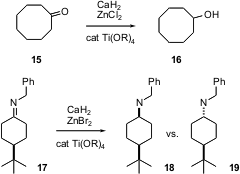
Commonly, the inexpensive CaH2 is used as a drying agent. Sentaro Okamoto of Kanagawa University, Yokohama, has found (Tetrahedron Lett. 2005, 46, 1667.DOI: 10.1016/j.tetlet.2005.01.058)that in the presence of a stoichiometric amount of ZnX2 and a catalytic amount of Lewis acid, ketones such as 15 are readily reduced to the corresponding alcohol, and imines such as 17 are reduced to the amine. The reduction of the cyclic imine 17 itself was not reported, but it is included here because the stereochemical outcome is of substantial importance. While there are many ways to reduce the imine of a cyclic ketone to the axial amine 18, there is as yet no general way to reduce it to the equatorial amine 19.