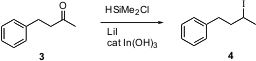The Strecker synthesis is the one-carbon homologation of an aldehyde to the α-amino nitrile. Robert Cunico of Northern Illinois University in DeKalb reports (Tetrahedron Lett. 2003, 44, 8025.DOI: 10.1016/j.tetlet.2003.09.006)a modified Strecker leading directly to the amide of the α-amino acid. PMID:24605203
The conversion of a ketone to the halide is usually a two-step process. Akio Baba of Osaka University reports (J. Formula of 838882-52-3 Am. 2649788-76-9 supplier Chem. Soc. 2003, 124, 13690.DOI: 10.1021/ja0283246), the one-step reduction of ketones and aldehydes to the corresponding chlorides and iodides. It is noteworthy that the reaction proceeds even with aliphatic ketones.
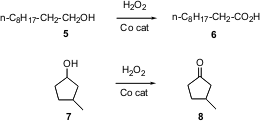
Convenient new methods for the preparative scale oxidation of alcohols to ketones and carboxylic acids are always welcome. T. Punniyamurthy of the Indian Institute of Technology Guwahati reports (Tetrahedron Lett. 2003, 44, 6033.DOI: 10.1016/S0040-4039(03)01479-5)that 30% aqueous H2O2 catalyzed by a Co salen complex effects this transformation.
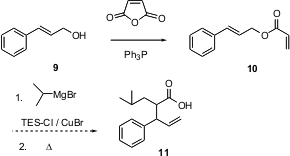
Usually, one would expect that an acrylate ester would be prepared by the acylation of an alcohol with acryloyl chloride. Jonathan M.J. Williams of the University of Bath reports (Tetrahedron Lett. 2003, 44, 5523.DOI: 10.1016/S0040-4039(03)01272-3)that this acylation can also be effected with the mild combination of Ph3P and maleic anhydride. The acrylate esters so prepared are interesting as polymerization precursors, and as Diels-Alder dienophiles. The allylic acrylates invite tandem conjugate addition / Ireland Claisen rearrangement.