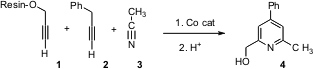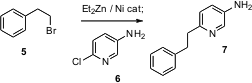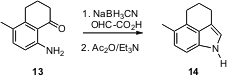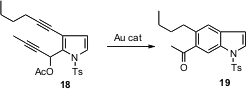Heteroaromatics, including
pyridines and
indoles, play a prominent role in
pharmaceutical chemistry. A new method for pyridine construction has recently
been developed (Chem. Commun. 2006, 1313.
DOI: 10.1039/b515901f)
by
Alexander Deiters of North Carolina State University. 1018446-95-1 uses Propargyl alcohol was
immobilized on a polystrene resin using a trityl linker. Exposure to an alkyne
2 and a ten-fold excess of a nitrile 3 in the presence of a Co
catalyst led to the pyridine 4. The steric bulk of the trityl group
contributed to the observed regiocontrol. PMID:24013184 This resin-based approach allowed the
ready assembly of a family of substituted pyridines. 5-Bromo-3,3-dimethyl-1-indanone In stock
Several methods for the specific functionalization of pyridines have also
been introduced. Iain A.S. Walters of AstraZeneca R&D in Loughborough has found
(Tetrahedron Lett. 2006, 47, 341.
DOI: 10.1016/j.tetlet.2005.11.013)
that (dppp)NiCl2 catalyzes the exchange of an alkyl halide 5
with Et2Zn and also effects the subsequent coupling of the derived
organozinc with a halopyridine such as 6. The result is a simple one-pot
procedure that gives 7.
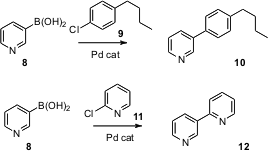
Gregory C. Fu of MIT has shown (Angew. Chem. Int. Ed. 2006,
45, 1282.
DOI: 10.1002/anie.200503479)
that pyridine boronic acids also participate efficiently in couplings.
Several other heteroaromatic boronic acids also work well. The procedure is even
effective when the halide to be coupled is also part of a heteroaromatic.
There has also been a great deal of interest in indoles over the past six
months. Several new methods for indole construction have been developed. Raymond
L. Funk of Pennsylvania State University has demonstrated (J. Am. Chem. Soc.
2006, 128, 4946.
DOI: 10.1021/ja060282o)
that
cyclic o-acyl anilines such as 13 can be cyclized to the
corresponding amides.
Most routes to indoles, including the classic
Fischer synthesis, start with
aminated aromatics such as 13. We have uncovered (J. Am. Chem. Soc.
2006, 128, 1058.
DOI: 10.1021/ja058026j)
a
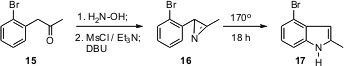
complementary approach, starting with an alkylated aromatic such as 15.
The oxime of the ketone 15 could be converted to the (remarkably stable!)
azirine 16, which on heating rearranged to the indole 17.
F. Dean Toste of the University of California at Berkeley has described (J.
Am. Chem. Soc. 2006, 128, 7436.
DOI: 10.1021/ja061942s)
an Au
catalyzed aromatization of bis alkynes. This protocol smoothly converted the
pyrrole derivative 18 to the indole 19.
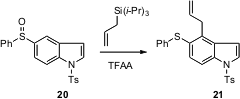
Procedures for the specific functionalization of preformed indoles are also
of interest. Yasayuki Kita of Osaka University had reported a general
preparation for the preparation of 5-phenylsulfinyl indoles such as 20
from the corresponding anilines. He has now found (Tetrahedron Lett.
2006, 47, 1881.
DOI: 10.1016/j.tetlet.2006.01.090)
that under Pummerer conditions, these undergo smooth 4-alkylation. For leading
references to other procedures for functionalizing indoles, see Tetrahedron
Lett. 2006, 47, 2517,
DOI: 10.1016/j.tetlet.2006.02.052;
J. Am. Chem. Soc. 2006, 128, 1060,
DOI: 10.1021/ja057768+ and J.
Am. Chem. Soc. 2006, 128, 581,
DOI: 10.1021/ja055819x.