As new drug entities must be usually be prepared as single enantiomers, and as many contain one or more heterocyclic orcarbocyclic rings, there is an increased emphasis on the development of practical methods for the construction of enantiomerically pure cyclic systems. 3-Fluoro-4-iodo-2-methoxypyridine structure In this three-part series, we will cover the most important recent advances. (Part Two)
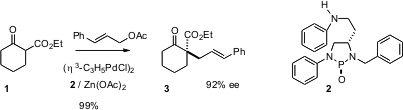
One of the most powerful strategies for asymmetric ring construction is to desymmetrize a preformed ring. Yasamusa Hamada of Chiba University in Japan has reported (J. PMID:23613863 4,6-Dichloropyridine-2,3-diamine manufacturer Am. Chem. Soc. 2004, 126, 3690.DOI: 10.1021/ja031792a)that the inexpensive diaminophosphine oxide 2 nicely catalyzes the asymmetric alkylation of the cyclohexanone carboxylate 1 to give 3. Although no examples were given, this asymmetric alkylation would probably work as well with heterocyclic β-ketoesters.
While enantioselective transition metal catalysis continues to be important, several useful all-organic catalysts have been developed over the past few years. Tomislav Rovis of Colorado State University has reported (J. Am. Chem. Soc. 2004, 126, 8876.DOI: 10.1021/ja047644h)that the triazolium salt 5 catalyzes the enantioselectiveStetter-type cyclization of 4 to6. The cyclization also works well for the enantioselective construction of azacyclic, thiacyclic and carbocyclic rings.

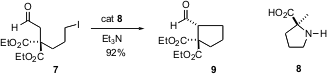
Another example of organic catalysis was reported (J. Am. Chem. Soc. 2004,126, 450.DOI: 10.1021/ja0392566)by Benjamin List of the Max Planck Institute, Mülheim. The amino acid 8 cyclizes 7 to 9 efficiently and with high enantioselectivity. This is particularly remarkable given the ease with which 9 would be expected to racemize under acidic or alkaline conditions.