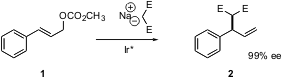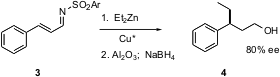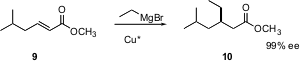There are several strategies now available for the catalytic enantioselective construction of oxygenated and aminated stereogenic centers. Catalytic processes for the enantioselective construction of alkylated stereogenic centers are just starting to appear. In general, investigators have approached this problem by reacting a prochiral electrophile with a chiral catalyst and a nucleophile. PMID:24103058 There are three variables that are useful to consider: the nucleophile, the catalyst, and the substituents at the reacting center. 2-Amino-2-thiazolin-5-one Price
One of the first such reactions to be reduced to practice was the addition of malonate to cinnamyl carbonate 1. The current state of the art is illustrated by a recent contribution (Org. Lett. 2005, 7, 1621. DOI: 10.1021/ol050350w)from Alexandre Alexakis at the University of Geneva, in which the Ir catalyst was further optimized. 6-Bromo-2(1H)-quinolinone manufacturer The coupling proceeded with 98:2 regioselectivity in the desired sense.
In general, aryl substituted stereogenic centers such as that of 2 are the easiest to form with high enantioselectivity. Thus, when Kiyoshi Tomioka and co-workers of Kyoto University investigated (J. Org. Chem. 2005, 70, 297. DOI: 10.1021/jo0484225)the conjugate addition of dialkyl zinc reagents to α,β-unsaturated aldehydes, they began with substrates such as 3. They found that when the aldehyde was replaced by a sterically-demanding sulfonyl imine, Cu*-mediated conjugate addition was efficient. They chose to analyze the intermediate enantiomerically-enriched aldehyde as the corresponding alcohol 4. The ee’s are modest by current standards.
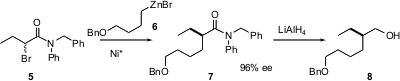
Alkyl zinc halides are more readily available than dialkyl zincs. Gregory C. Fu of MIT recently described (J. Am. Chem. Soc. 2005, 127, 4594. DOI: 10.1021/ja0506509)the Ni*-mediated coupling of such reagents to racemic α-bromoamides such as 5, to give the highly enantiomerically-enriched products such as 7. This is the functional equivalent of catalytic enantioselective alkylation of the amide enolate. The amide 7 is easily reduced to the primary alcohol 8. The authors note that the alkyl zinc halides must be freshly prepared – a commercial reagent did not work.
Conjugate addition is complementary to α-alkylation. Ben L. Feringa of the University of Groningen has described (Angew. Chem. Int. Ed. 2005, 44, 2752. DOI: 10.1002/anie.200500317)the catalytic enantioselective addition of Grignard reagents to α,β-unsaturated esters such as 9. This procedure works well with a wide range of esters and Grignard reagents.
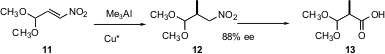
Some trialkyl aluminum reagents are commercially available. Alexandre Alexakis of the University of Geneva has also reported (Tetrahedron Lett. 2005, 46, 1529. DOI: 10.1016/j.tetlet.2005.01.007)the conjugate addition of Me3Al to nitro alkenes such as 11. The product nitro compounds can be converted into acids without racemization. Carreira previously ( 2005, June 20) has shown that nitro compounds such as 12 can also be converted into the corresponding nitriles without epimerization.
2005, June 20) has shown that nitro compounds such as 12 can also be converted into the corresponding nitriles without epimerization.
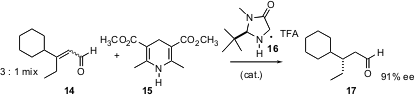
One could imagine that alkylated stereogenic centers could also be constructed by the enantioselective reduction of trisubstituted alkenes. David W. C. MacMillan of Caltech has now (J. Am. Chem. Soc. 2005, 127, 32. DOI: 10.1021/ja043834g)reduced this to practice. Using Hantsch ester 15 and an organocatalyst, geometric mixtures of α,β-unsaturated aldehydes such as 14 are reduced with remarkable enantiomeric excess.