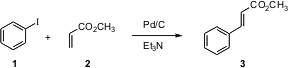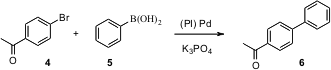Two of the most common methods for homologating an aryl halide are the Heck reaction (1 -> 3) and the Suzuki reaction (4 -> 6). Shriniwas D. Samant of the University of Mumbai has reported (Tetrahedron Lett. 2005, 46, 2483.DOI: 10.1016/j.tetlet.2005.02.035)optimization of the conversion of 1 to 3 using Pd/C as the catalyst, at room temperature with ultrasound acceleration. 2090927-90-3 Formula Formula of 6-Chloro-1H-pyrazolo[3,4-b]pyridine The reaction proceeded most efficiently using NMP as the solvent, and Et3N as the base.
For the Suzuki coupling of 4 plus 5 to give 6, Shu Kobayashi of the University of Tokyo has been investigating (Org. PMID:23659187 Lett. 2005, 7, 4831.DOI: 10.1021/ol051526x)polymer-incarcerated (PI) Pd. There is no need for added ligand, as the incarcerating polymer includes triaryl phosphines. The Pd did not leach at all into the reaction solution, so it could be used multiple times with no diminution in yield.
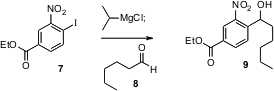
Nitro-substituted organometallics are notoriously difficult to prepare. Paul Knochel of the University of Munich has now shown (J. Org. Chem. 2005,70, 2445. DOI: 10.1021/jo048132o)that the method his group developed for halide exchange works well even with nitro iodides such as 7.
There is growing interest in methods for the direct functionalization of C-H bonds, including aromatic C-H bonds. Kyoko Nozaki of the University of Tokyo has found (Tetrahedron Lett. 2005, 46, 959.DOI: 10.1016/j.tetlet.2004.12.027)that the previously-reported Pd-mediated carboxylation of benzene derivatives proceeds much more efficiently in the presence of a phosphenium salt. Steric effects are pronounced, so homologation of 10 gives predominantly 11.
Functional groups can direct the metal-mediated C-H activation. Milton R. Smith III of Michigan State University found (J. Am. Chem. Soc. 2005,127, 10539. DOI: 10.1021/ja0428309)that the cyano group is a particularly powerful director of ortho metalation and functionalization.
More highly substituted benzene derivatives can be constructed by intramolecular bond formation. Rick L. Danheiser of MIT has shown (J. Am. Chem. Soc. 2005, 127, 5776.DOI: 10.1021/ja051180l)that the thermal cyclization of ynamides with conjugated enynes proceeds smoothly.
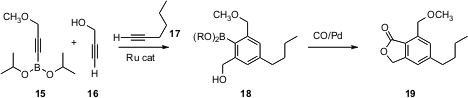
It is also possible to bring the ring components into proximity with a temporary tether. Yoshihiko Yamamoto of Nagoya University has developed (J. Am. Chem. Soc. 2005, 127, 9625.DOI: 10.1021/ja051377d)a boron tether that serves well. The product arylboronates such as 18 can then be further functionalized, as illustrated by carbonylation of 18 to give the phthalide 19. The alcohol 16 can be replaced by an enantiomerically-pure secondary propargyl alcohol, leading to the enantiomerically-pure secondary phthalide.