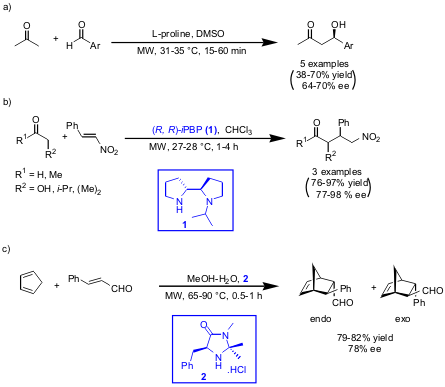
Sarah Mossé and Alexandre Alexakis from the University of Geneva have
reinvestigated asymmetric aldol (a),
Mannich (b) and
Diels-Alder reactions (c)
applying organocatalysis under microwave conditions (Org. PMID:23912708 Lett. BuyRuPhos Pd G3 2006,
8, 3577.
DOI: 10.1021/ol0614727). L-proline (a), N–i-Pr-2R,2’R-bipyrrolidine ((R, R)-iPBP,
1, b) and
McMillan imidazolidinone (2, c) were used as organocatalysts. In all cases the
reaction times were reduced dramatically using microwave heating. Additionally,
for the aldol (a) and Mannich reaction (b) catalyst loadings could be decreased
maintaining the enantioselectivities achieved by conventional rt experiments.
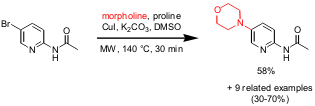
Cu-Catalyzed Aminations
The amination of various functionalized heterocyclic bromides with aliphatic
primary and cyclic amines and
pyrazole was disclosed by Vince S. C. Yeh and Paul
E. Methyl 7-bromo-1H-indole-6-carboxylate Data Sheet Wiedeman from Abbott Laboratories (Tetrahedron Lett. 2006, 47, 6011.
DOI: 10.1016/j.tetlet.2006.06.119).
Optimization studies have shown that high conversions to the desired product
could be achieved with the catalyst system CuI/proline which proved to be very
general in the further process. However, a rather high catalyst (20%) and ligand
(40%) loading was necessary to achieve the aminated products in moderate to good
yields.
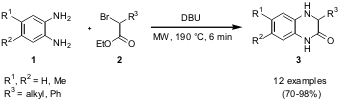
Synthesis of Quinoxalinones
Sukanta Kamila and Edward R. Biehl from the Southern Methodist University,
Dallas, have reported on the synthesis of potentially biologically active
quinoxalin-2-ones (Heterocycles 2006, 68, 1931.
DOI: 10.3987/COM-06-10793).
Symmetrical 1,2-diaminobenzenes 1 were reacted with ethyl 2-bromoalkyl/phenyl acetates
2 and DBU as base under solvent-free microwave conditions to give quinoxalinones
3 in good to excellent yields.
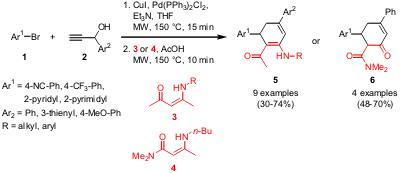
Synthesis of 1-Acetyl-2-amino-cyclohexa-1,3-dienes
Oana G. Schramm and Thomas J. J. Müller from the University of Heidelberg have
reported on the one-pot reaction of electron-deficient (hetero)aryl bromides 1,
(hetero)aryl propargyl alcohols 2 and enamino carbonyl compounds
3 or 4,
respectively (Synlett 2006, 1841.
DOI: 10.1055/s-2006-947352). The reaction proceeds via a
coupling-isomerization-enamine addition-aldol condensation sequence and
furnishes dienes 5 when enaminones 3 are used or the hydrolyzed products
6 if β-amino
crotonamide (4) is applied. Compared to thermal heating where dihydropyridines
are obtained, an aldol-type condensation is favored in the second step under
microwave conditions providing cyclohexa-1,3-dienes 5 and cyclohexanones
6, respectively.