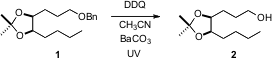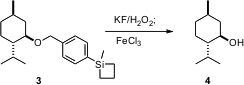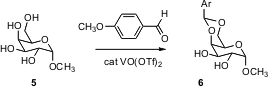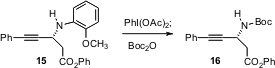Groups
The DDQ cleavage of a benzyl ether is a classic transformation, yet it has
not always been reliable. Kazunobu Toshima of Keio University has recently (Tetrahedron
Lett. 2005, 46, 7307.
DOI: 10.1016/j.tetlet.2005.08.132)
found that the efficiency of the deprotection, e.g. conversion of 1 to
2, is substantially improved if the reaction is exposed to long wavelength
UV. With acid sensitive substrates, yields are improved by the inclusion of
insoluble BaCO3. PMID:24367939 The previously observed variability in this
deprotection may be due to differences in ambient laboratory lighting. 1885090-83-4 Chemscene
One way to avoid the difficulties of benzyl deprotection has been to use the
more easily oxidized p-methoxybenzyl (PMB) group. 887144-97-0 custom synthesis Gregory B. Dudley of
Florida State University has developed (Tetrahedron Lett. 2005,
46, 3283.
DOI: 10.1016/j.tetlet.2005.03.110)
a group that is orthogonal to p-methoxy benzyl, based on a p-siletanyl
group (PSB). Mild conditions convert 3 to the p-hydroxybenzyl
ether, which is removed very quickly with FeCl3. In competition
experiments, deprotection of PMB could be carried out in the presence of PSB,
and the deprotection of PSB could be carried out in the presence of PMB.
Protection of 1,2- and 1,3-diols is also important. Chien-Tien Chen of
National Taiwan Normal University and Chung-Cheng Lin of the Academia Sinica,
Taipei have found (Org. Lett. 2005, 7, 3343.
DOI: 10.1021/ol051178z)
that
arylidene protection can be effected by direct condensation of an aromatic
aldehyde with the diol (e.g. 5) in the presence of catalytic vanadyl
triflate. This observation will make arylidene protection, especially with less
common aryl groups, more readily available.
Fernando Sartillo-Piscil of the Universidad Autónoma de Puebla, Mexico, has
uncovered (J. Org. Chem. 2005, 70, 7107.
DOI: 10.1021/jo050753+)
a new
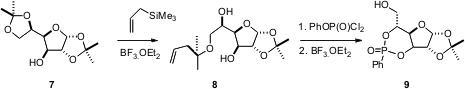
protocol for the selective deprotection of 1,2-diols. Exposure of 7 to
allyltrimethyl silane in the presence of BF3. Et2O
yields the monoadduct 8. After phosphorylation, the alcohol protecting
group is readily removed by exposure to BF3. Et2O,
to deliver 9.
Ester hydrolysis can present difficulties if there are sensitive functional
groups elsewhere in the molecule. Uwe T. Bornscheuer of Greifswald University
and George Kokotos of the University of Athens have found (J. Org. Chem.
2005, 70, 3737,
DOI: 10.1021/jo050114z; 8730,
DOI: 10.1021/jo051004v.) that
some readily-available enzymes can remove t-butyl, methyl and benzyl
esters, in the presence of amine protecting groups such as Boc, Z, and Fmoc.
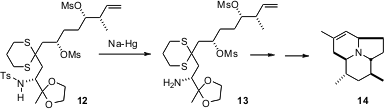
There is a widespread perception that the
N-tosyl group is difficult
to remove. In fact, it is often easily removed reductively. In the course of a
synthesis of the Dendrobatid alkaloid 205B (14), Amos B. Smith III of the
University of Pennsylvania demonstrated (Org. Lett. 2005, 7,
3247.
DOI: 10.1021/ol0510264)
that the N-tosyl group could be reductively removed under conditions that
did not affect two other organosulfur functional groups, mesylate and dithiane.
The 4-methoxyphenyl N-protecting group has often resisted efficient
oxidative removal (J. Org. Chem. 2005, 70, 10592.
DOI: 10.1021/jo051867o). Marc L.
Snapper and Amir H. Hoveyda of Boston College have now shown (Org. Lett.
2005, 7, 2711.
DOI: 10.1021/ol050910r)
that the
alternative 2-methoxyphenyl N-protecting group can be removed cleanly by
exposure to iodosobenzene diacetate.