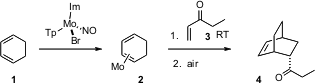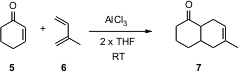Cyclic dienes such as 1 are reluctant participants in
Diels-Alder
cycloaddition. W. Dean Harman of the University of Virginia has shown (J. Am.
Chem. Soc. PMID:24761411 2006, 128, 1426.
DOI: 10.1021/ja0553654)
that such dienes are
activated by complexation to Mo. After cycloaddition, exposure of the product to
air liberates the product 4.
Cyclohexenone 5 is a sluggish dienophile, requiring activation by AlCl3.
Francesco Fringuelli and Ferdinando Pizzo of the Università di Perugia have
developed (Org. Lett. 1421473-07-5 web 2006, 8, 2487.
DOI: 10.1021/ol060569q)
a somewhat milder
alternative, AlCl3 complexed to THF. Operationally, a catalytic
amount (5 mol %) of AlCl3 and exactly twice that much THF were
combined. The diene 6 and the dienophile 5 were then added, in
this case in a 2:1 ratio, without additional solvent. After 12 hours at 30 °C,
the cycloadduct 7 was isolated in 80% yield. Buy85272-31-7 Increasing or decreasing the
amount of THF dropped the efficiency of the reaction.
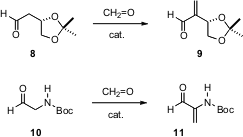
Effective procedures for the α-methylenation of aldehydes go back at least sixty years
(J. Am. Chem. Soc. 1948, 70, 1694.
DOI: 10.1021/ja01185a006).
Nevertheless, the results reported (J. Org. Chem. 2006, 71, 2538.
DOI: 10.1021/jo052529q)
by Petri M.
Pihko of Helsinki University of Technology are compelling. Using 37% aqueous
formaldehdye and 10 mol % of the dimeric peptide L-Pro-β-Ala, they were able to
effect α-methylenation of sensitive aldehydes such as 8 and 10.
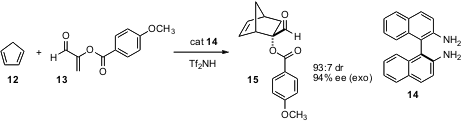
α-Substituted acroleins are difficult substrates for enantioselective
catalysis. Kazuaki Ishihara of Nagoya University has developed polyamine
catalysts that direct cycloaddition of α-substituted acroleins with high
enantioselectivity. For cyclopentadiene 12 and the dienophile 13 (Org. Lett.
2006, 8, 2229.
DOI: 10.1021/ol060490l), the polyamine of choice is
14, 5 mol %, activated with an
equimolar amount of Tf2NH.
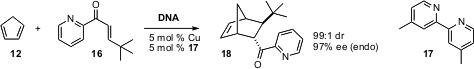
Ben L. Feringa and Gerard Roelfes of the University of Groningen have taken
advantage (Chem. Comm. 2006, 635.
DOI: 10.1039/b516552k)
of the inherently chiral environment of DNA to
direct the absolute sense of Diels-Alder cycloaddition.
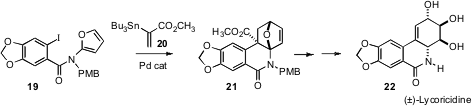
Two particularly elegant applications of the intramolecular Diels-Alder
reaction have recently been reported. Albert Padwa of Emory University has shown
(Org. Lett. 2006, 8, 247.
DOI: 10.1021/ol052524f)
that Pd-mediated coupling of
19 and 20 directly
delivers the cycloadduct 21. This was carried on over several steps to the
Amaryllidaceae alkaloid lycoricidine 22.
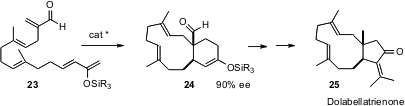
E.J. Corey of Harvard University has applied (J. Am. Chem. Soc. 2006,
128,
740.
DOI: 10.1021/ja0576379)
the chiral Diels-Alder catalysts he has developed to the cyclization of the
triene 23. The adduct 24 was carried on to the dolabellane marine natural
product dolabellatrienone 25.