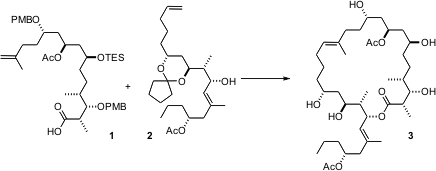
The macrolides dolabelides A-D, isolated from the sea hare Dolabella, are cytotoxic against HeLa-S3 cells at concentrations of 1.3 – 6.3 μg/mL. The recent (J. Am. PMID:25105126 Chem. Soc. 6-Bromothiazolo[4,5-b]pyridin-2-amine site 2006, 128, 2796.DOI: 10.1021/ja058692k)synthesis of dolabelide D (3) by James L. Leighton of Columbia University nicely highlights the powerful reagent-based methods for acyclic stereoselection that they have recently developed.
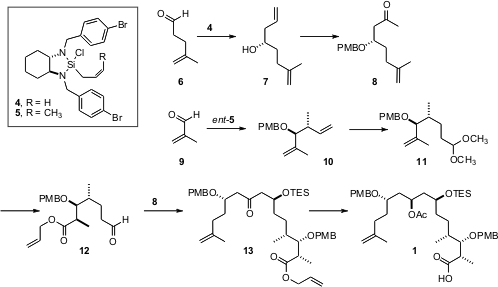
Two such reagents, easily prepared on a large scale, are the amino silanes 4 and 5. Tributyl(1-ethoxyethenyl)stannane uses These were used to prepare 8 and 12, which were combined to prepare1. Homologation of the aldehyde 6 with 4 gave 7. Protection followed byWacker oxidation then delivered 8. To prepare 12, methacrolein 9 was homologated withent–5. Hydroformylation followed by in situ acetal formation gave11. Diastereroselective hydroboration followed by oxidation and esterification then led to 12. Aldol condensation of 12 with 8, taking advantage of the inherent chirality of the alkoxy enolate, proceeded to give, after protection, a 10:1 ratio of 13 and its diastereomer.
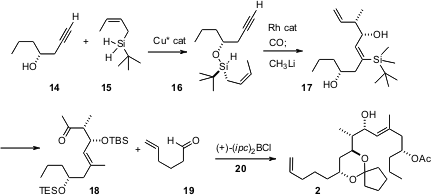
The preparation of alkene 2 depended on a third reagent the Leighton group has developed, the silane 15. Enantioselective condensation of 14 with15 set the absolute configuration around Si. In the next step, intramolecular carbonylative silylation followed by intramolecular crotylation of the transient aldeyde, the absolute configuration at the Si in 16 directed the new stereogenic centers of 17. To achieve the requisite diastereoselectivity in the aldol condensation of the derived ketone 18 with the aldehyde 19, the enolate of 18 was prepared using the chiral director 20.
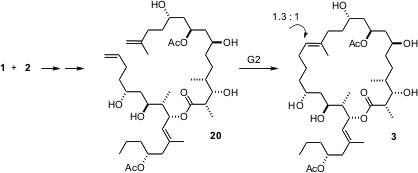
To complete the synthesis, it was necessary to form the lactone between 1 and 2, and also to effect alkene metathesis. The esterification proceeded smoothly, to give, after functional group deprotection, the linear precursor 20. Alkene metathesis was efficient, but proceeded with little geometric control. It would have been interesting to know what influence the several protecting groups might have had on the geometry of the metathesis step.