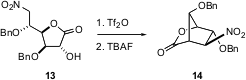New methods are being developed for the enantioselective construction of both heterocyclic and carbocyclic rings. Justin Du Bois of Stanford reports (J. Formula of 1310405-06-1 Am. Chem. Soc. 2003, 125, 2028.DOI: 10.1021/ja028916o)that his intramolecular Rh-mediated nitrene C-H insertion cyclizes 1 to 2 with high diastereoselectivity. PMID:24487575 The N,O-acetal opens smoothly with the alkynyl zinc, again with high diastereocontrol. The tosylate is stable to the alkyne addition conditions, but after reduction of the alkyne to the alkene the tosylate is readily displaced, to give4. 5-Bromo-2-chloropyridin-4-ol Order After osmylation, the sulfamate undergoes smooth SN2 displacement with cyanide ion, leading, after reduction, to the indolizidine6.
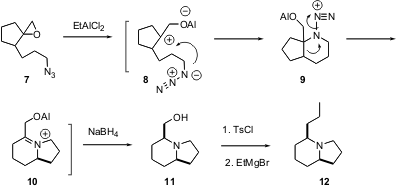
Sundarababu Baskaran of IIT-Madras offers (Org. Lett. 2003, 5, 583.DOI: 10.1021/ol027563v)an alternative route to indolizidines. Exposure of the epoxide 7 to Lewis acid followed by reduction leads to 11 as a single diastereomer. The authors hypothesize that this rearrangement is proceeding via intermediates 8 – 10. Tosylation of 11 followed by homologation leads to the Dendrobatid alkaloid12.
Intramolecular carbon-carbon formation to convert inexpensive, enantiomerically-pure carbohydrates directly to highly functionalized, enantiomerically-pure carbocycles has long been a goal of organic synthesis. Ramón J. Estévez of the University of Santiago in Spain reports (Org. Lett. 2003, 5, 1423.DOI: 10.1021/ol034127f)that TBAF smoothly converts the triflate derived from 13 into 14, without competing β-elimination.