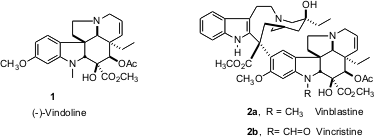
The Vinca-derived vinblastine (2a) and vincristine (2b) are still mainstays of cancer chemotherapy. Price of 1662706-59-3 The more complex half of these dimeric alkaloids, vindoline (1), has in the past presented a formidable challenge for total synthesis. PMID:26760947 Dale L. 4,6-Dichloropyrimidin-5-amine Chemscene Boger of Scripps, La Jolla has developed (Org. Lett. 2005, 7, 4539. DOI: 10.1021/ol051975x)a strikingly simple solution to this problem, based on sequential cycloaddition.
The starting point for the synthesis was N-methyl 6-methoxytryptamine (3), an improved preparation of which is described by the authors. This was extended to 4, which was then cyclized to 5, and acylated with 6 to give 7. On heating, 7 cyclized to 8, which lost N2 to give the zwitterion 9. Addition of the intermediate 9 to the indole then gave 10. In one reaction, the entire ring system of vindoline, appropriately oxygenated, was assembled! The precursor 7 was flat, so it offered no opportunity for chiral synthesis. Fortunately, 10 and ent–10 proved to be very easy to resolve by chiral chromatography.
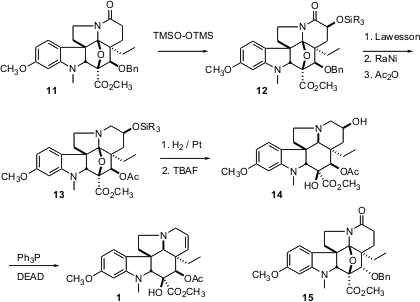
To complete the synthesis, the δ-lactam 11 was oxygenated to 12. Conditions for desulfurization of the derived thiolactam also effected debenzylation, to give, after acetylation, the ether 13. Pt-mediated hydrogenolysis gave 14, which was dehydrated to vindoline (1).
A complementary synthetic route, based on the E isomer of 6, and so leading through the ether 15, is also described. Although slightly longer, this approach was about as efficient as the route via 11.