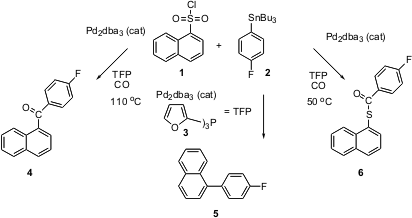
Many aryl coupling reactions have been carried out on bromides, but often the much more expensive aryl triflates are required. Pierre Vogel of the Swiss Institute of Technology in Lausanne has carried out (J. Am. Chem. Soc. PMID:23710097 2003, 125, 15292.DOI: 10.1021/ja038328q)a detailed investigation of the Stille coupling with a series of inexpensive arenesulfonyl chorides, including1. In addition to carbonylative coupling, to give 4, and non-carbonylative coupling, to give5, the reaction can be directed toward the thioester 6. 821785-75-5 Order This is a new and potentially very useful procedure for reducing an arenesulfonate to the protected thiophenol. Fludioxonil In stock
Alkynes are usually alkylated under strongly basic conditions. Gregory Fu of MIT recently reported (J. Am. Chem. Soc. 2003, 125, 13642.DOI: 10.1021/ja038177r)a much milder Pd and Cu mediated coupling, illustrated by the reaction of the terminal alkyne 7 with the alkyl iodide 8 to give 9. Alkyl bromides work equally well. It is exciting that ketones, esters, alkyl chlorides, alkenes, nitriles and acetals are compatible with the procedure. The key to the reaction is the use of the supporting carbene ligand10.
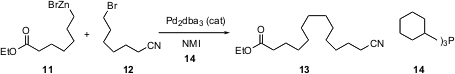
Professor Fu has also developed (J. Am. Chem. Soc. 2003, 125, 14726.DOI: 10.1021/ja0389366)an equally powerful Pd-mediated procedure for sp3– sp3 coupling. With 14 as the supporting phosphine, the organozinc bromide 11(easily prepared by the action of Zn metal on the bromide) couples with the bromide 12 to give 13. Chlorides and tosylates also serve efficiently as leaving groups, and ethers, amides and acetals are compatible with the coupling conditions. The organozinc halide and/or the coupling partner may also be sp2 hybridized. The coupling reaction is limited to the formation of primary sp3-hybridized bonds.