The indole scaffold is a prominent and privileged structural motif found in
numerous natural products and various synthetic compounds. N-(Azido-PEG3)-N-(PEG2-NH-Boc)-PEG3-acid Formula Recently, a large
number of indole-containing compounds have revealed remarkable pharmacological
activity and their utility as therapeutic agents has attracted considerable
attention from chemists (Angew. Chem. 2005, 44, 606,
DOI: 10.1002/anie.200461864;
J. Am. Chem. Soc. (3R)-3-Methylpyrrolidin-3-ol site 2005,
127, 5342,
DOI: 10.1021/ja0510616). Libraries based on the indole scaffold have been developed to
address the need for novel drugs with increased potency (J. Comb. Chem.
2005, 7,
130,
DOI: 10.1021/cc049922e;
Tetrahedron 2006, 62, 3439,
DOI: 10.1016/j.tet.2006.01.047). Subsequently, the development of efficient
methods that allow rapid access to functionalized indoles with different
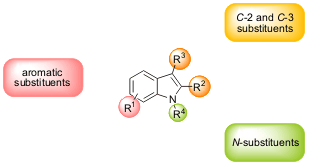
substitution patterns (at C-2, C-3, N-atom and aromatic ring, Figure 1)
constitutes an emerging area.
The most recent advances on catalytic methods to achieve the synthesis of
substituted indole are highlighted herein. For a review on earlier work on
indole synthesis, see J. Chem. Soc. Perkin Trans. 1 2000, 1045.
DOI: 10.1039/a909834h)
I. Palladium-catalyzed methods
Senanayake and co-workers of the Boehringer-Ingelheim Pharmaceuticals
(Virginia) (Org. Lett. PMID:23381601 2006, 8, 3271.
DOI: 10.1021/ol061136q)
have reported on a one-pot,
three-component procedure for the synthesis of 2,3-substituted indoles based on
Cacchi´s protocol (Scheme 1).
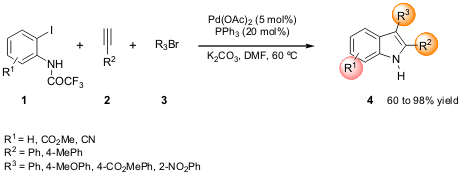
Scheme 1 – One-pot, three-component procedure for accessing
the 2,3-substituted indole scaffold.
This regiospecific procedure consisted of a Pd domino indolization involving
a consecutive Pd-catalyzed Sonogashira coupling followed by aminopalladation and
reductive elimination starting from 2-iodo-N-trifluoroacetylanilide 1, a suitable acetylene
2 and bromoarene 3. The Senanayake group optimized the
reaction conditions as shown in Scheme 1: the use of trifluoroacetyl as
protecting group in 1 was shown to be advantageous (readily hydrolyzable);
addition of bromobenzene at the beginning simplified the procedure and enhanced
the reaction rate; DMF as solvent combined with K2CO3 as base, and a temperature
of 60 ºC gave better results.
The Larock group of the Iowa State University reported (J. Org. Chem.
2006,
71, 62.
DOI: 10.1021/jo051549p)
on the synthesis of 3-iodoindoles 8 via Pd/Cu-catalyzed coupling of
N,N-dialkyl-2-iodoanilines 5 with terminal acetylenes 6 and subsequent
electrophilic cyclization of 7 (Scheme 2). Due to the high reactivity of
N,N-dialkyl-o-iodoanilines towards the
Sonogashira coupling, a wide variety of
substituted anilines and alkynes were used (with aryl, vinyl, alkyl and silyl
groups). However, the authors reported that substituents on the triple bond of
7 affect the yield of the following cyclization step, since increased conjugation
enhances the reaction rate and also increases the product yield. In addition,
while electron-withdrawing groups enhanced cyclization, (strong)
electron-donating groups slowed the reaction and led to lower yields.
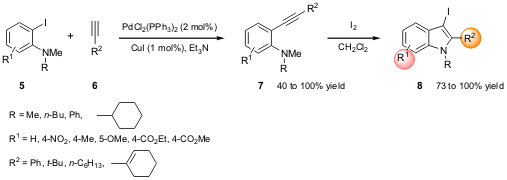
Scheme 2 – Synthesis of 3-iodoindoles by Pd/Cu-catalyzed
coupling followed by electrophilic cyclization.
The authors reported an interesting feature; when there are two different
N-alkyl groups, the less-hindered group is more easily removed. Additionally,
this procedure allows further derivatization/functionalization at C-3, since 8 might be used for cross-coupling reactions.
The Lautens group of the University of Toronto described a modular synthesis
of 2-substituted indoles via a palladium-catalyzed coupling (Org. Lett.
2005, 7,
3549.
DOI: 10.1021/ol051286l). This methodology involved an intramolecular
Buchwald-Hartwig C-N/intermolecular
Suzuki-Miyaura C-C coupling of o-gem-dihalovinylanilines with an organoboron
reagent catalyzed by Pd(OAc)2/S-Phos in the presence of K3PO4.H2O (Scheme 3).
Interestingly, the free aniline furnished the desired indole directly and in
good yield. The strategy reported by the authors showed a wide range of
applicability in terms of substituents, in particular for the challenging
4-substituted indoles. Better results were obtained for X = Cl. Moreover, the
authors expanded the reaction scope to obtain 1,2,3-trisubstituted indoles by
switching the order of addition of the two boronic acids.
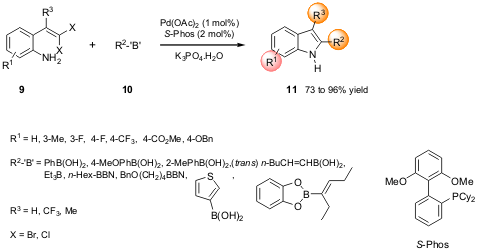
Scheme 3 – Synthesis of 2,3-disubstituted indoles using a
Pd-catalyzed C-N/C-C coupling strategy.
II. Zn(OTf)2 –Catalyzed Cyclization
The Liu group of the National Tsing-hua University published a new indole
synthetic approach (J. Org. Chem. 2006, 71, 4951.
DOI: 10.1021/jo0606711), using anilines
12 as starting material
with the appropriately substituted propargyl alcohols 13 as the
source of the C-2―C-3 unit. This method proved to be very effective in the
preparation of several indoles 14 in good to high yields (Scheme 4), since
Zn(OTf)2 has the advantage of activating not only the C-2-addition of the
alcohol but also the subsequent cyclization step. The mechanism elucidated by
the authors proposed that the isomerization of the α-amino ketone intermediate
occurs through a 1,2-nitrogen shift, thus explaining the observed
chemoselectivity.
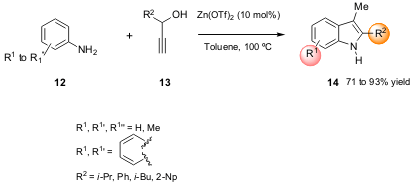
Scheme 4 – Zn(OTf)2-catalyzed cyclization of propargyl
alcohols 13 with anilines 12.
III. Rearrangement of Azirines via Thermolysis
D. Taber and W. Tian of the University of Delaware have reported on the
synthesis of indole 17 (J. Am. Chem. Soc. 2006, 128, 1058.
DOI: 10.1021/ja058026j)
via the thermal
rearrangement of azirines 16 that are readily available from the ketones
15 (Neber
reaction) via the corresponding activated oxime (Scheme 5). The rearrangement
occurred at temperatures ranging from 40 ºC up to 170 ºC. The authors suggest
that the cyclization mechanism proceeds by a π-participation of the aromatic
ring followed by reorganization, before the new C-N bond is formed.
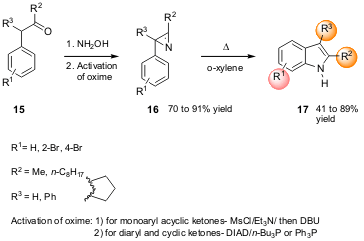
Scheme 5 – Indole synthesis via rearrangement of
functionalized azirines 16.
IV. N-Methoxyindoles via Alkylative Cycloaddition of Nitrosoarenes with Alkynes
The Nicholas and Penoni groups of the University of Oklahoma and of the
Università degli Studi dell’Insubria, respectively, have reported on a pathway
to N-methoxyindoles via an alkylative cycloaddition reaction (J. Org. Chem.
2006, 71, 823.
DOI: 10.1021/jo051609r).
The authors performed a one-pot procedure for the preparation of
several substituted N-methoxyindoles 20 using as starting materials the readily
available nitrosoarenes 18 and the alkyne 19 (Scheme 6). Both electron-poor and
electron-rich nitrosoarenes gave good product yields and regioselectivity for
the 3-position was observed. The authors obtained higher yields for
2-substituted nitrosoarenes 18 when compared to 4-substituted nitrosoarenes 18. Additionally, this method
constitutes a formal synthesis of the corresponding indole (NH) since the latter
can be formed by reduction of 20.
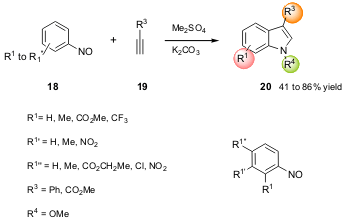
Scheme 6 – Synthesis of N-methoxyindoles 20 through
alkylative cycloaddition.
Recently, a review on the synthesis of indole derivatives via the versatile
isocyanides was reported by M. Gárcia-Valverde and T. Torroba groups of the
Universidad de Burgos and of the Università degli Studi di Firenze, respectively
(Org. Biomol. Chem. 2006, 4, 757.
DOI: 10.1039/b514946k).