Solid supports are frequently used in the separation of homogeneous ligands or catalysts from a reaction mixture. Buy1247542-90-0 There are two main methods to immobilize a chiral ligand on a support: (1) grafting of the desired ligand onto a preformed support containing reactive groups and (2) co-polymerization of a suitably functionalized ligand with polymerisable monomers and cross-linkers. Since there are many commercially available polymeric supports, the first method is often preferred. However, better control around the ligand within the polymer matrix is achieved using co-polymerization. An excellent review related to stereocontrolled diversity-oriented synthesis was recently published (Chem. Biol. , 2005, 12, 163. DOI: 10.1016/j.chembiol.2005.01.011). Enantioselective HTS has been explored in more depth over the last few years and this Highlight will provide an overview of the latest developments from 2005 to present.
1. Using preformed supports
Delpiccolo and co-workers carried out an asymmetric version of the solid-phase Staudinger reaction using different resin-bound aldimines 1 and a chiral auxiliary at C-3. Using the Evans procedure, the authors prepared the homochiral (S)-4-phenyloxazolidinylacetic acid (2) and the asymmetric Staudinger reaction was then performed on the solid support by adding (S)-(4-phenyloxazolidinyl) ketene (generated in situ by treating the crude acid chloride 3 with triethylamine at low temperature) to suspensions of 1, yielding the optically activeβ-lactams 4. Good to very good overall isolated yields (42-83%) were achieved with very high diastereoselectivity (up to 25/1 dr). (J. Comb. PMID:33679749 Chem. 2005, 7, 331. DOI: 10.1021/cc049825l).
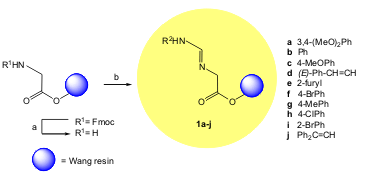
a) 30% piperidine in DMF. b) R2CHO (5 equiv), 1% v/v AcOH in DMF.
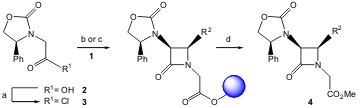
a) (COCl)2, toluene, 3 h, 60 °C. b) 1a-j, CH2Cl2, Et3N (20 equiv), 3 (15 equiv), 0 °C then rt overnight. c) 1a-e: CHCl3, 2 (2.5 equiv), Et3N (6 equiv), then 2-chloro-1-methylpyridinium iodide, r.t. 24 h. Buy149353-71-9 d) (i) 10% TFA in CH2Cl2. (ii) CH2N2.
Jew and co-workers developed a highly efficient enantioselective solid-phase synthetic methodology for unnatural (R)-amino acids using a phase-transfer catalytic alkylation of a resin-bound diphenylmethyleneglycinimine tert-butyl ester 5. The authors also found that by employing an aldimine linker, a high enhancement in the enantioselectivity is obtained. The solid-supported substrate5, is easy to prepare and the reaction proceeds under high enantioselectivity [86 to 99% ee, (S)-selective] and very mild conditions (J. Org. Chem. 2005, 70, 1904. DOI: 10.1021/jo0481957)
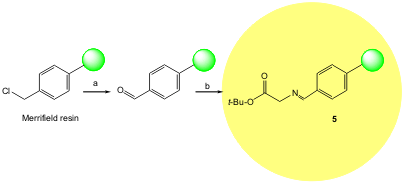
a) NaHCO3, DMSO, 150 °C, 12 h; b) glycine tert-butyl ester. HCl, Et2N, C6H6, reflux, 27 h.
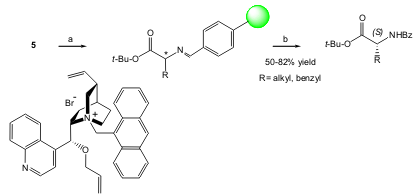
a) cat. (10 mol %), 50% aq-CsOH, RX, toluene/CHCl3 (7:3), 0 °C, 4 days; b) 1 N aq HCl, THF, 0 °C; c) BzCl, Et3N, CH2Cl2, 0 °C.
Panek and co-workers have developed an iterative resin capture-release strategy for the synthesis of stereochemically well-defined polypropionate arrays using anthracene-tagged organosilanes 6. The sequence involves enantioselective crotylation, followed by [4 + 2] cycloaddition-removal of the tagged product to afford a resin-bound adduct. Oxidative cleavage to afford an aldehyde may be followed by further iterations of crotylation, cycloaddition-removal, and oxidative release. This overall strategy, combining synthesis and purification, represents a specific application of resin-capture-release involving asymmetric synthesis of complex stereochemical arrays (Org. Lett. 2005, 7, 4435. DOI: 10.1021/ol0516723).
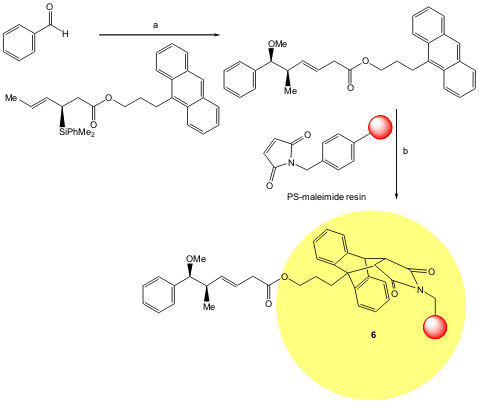
a) TMSOMe, TFOH, -78 °C, 4 h; b) MW (200 W), 150 °C, 15 min.
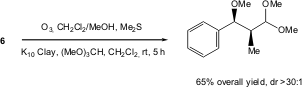
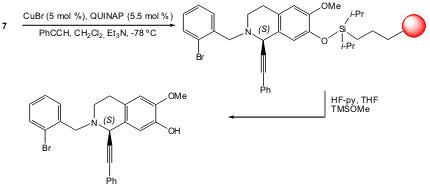
Polystyrene resin-bound alkyliminium 7 was used in the asymmetric addition of a terminal alkyne in the presence of triethylamine and catalytic copper bromide and QUINAP. Schreiber and Taylor reported that a highly pure (>90%) propargylic amine was obtained with high yield and good enantiomeric excess (84% overall yield, 75% ee) (Org. Lett. 2006, 8, 143. DOI: 10.1021/ol0526165).
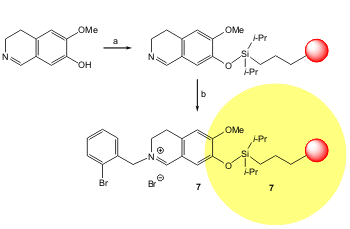
a) 500 μm polystyrene resin (macrobeads), CH2Cl2, 2,6-lutidine, DMF, rt, 20 h; b) 2-bromobenzyl bromide, Et2O.
Pericàs and co-workers have shown for the first time that click chemistry is a suitable strategy for immobilizing an hydroxyproline derivative on a Merrifield resin. The organocatalyst 8 promotes direct asymmetric aldol reactions in water with high diasteroselectivity (up to 98:2 syn/anti ratio). Aromatic aldehydes reacted with cyclohexanone with higher diastereoselectivity than that recorded for monomeric proline derivatives in water (Org. Lett. 2006, 8, 4653. DOI: 10.1021/ol061964j).
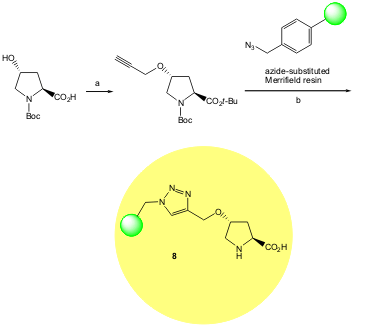
a) 1. t-BuBr, Et3NBn+Cl–, K2CO3, DMA, 55 ºC. 2. NaH, HCCCH2Br, DMF, -20 ºC to rt. b) 1. CuI (5 mol %), DIPEA. 2. TFA:CH2Cl2 1:1. 3. Et3N:THF 2:98.
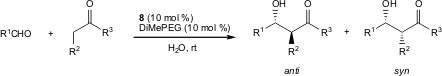
2. Using co-polymerization
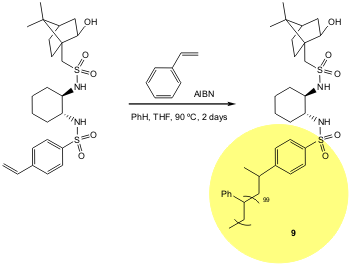
A simple and easy synthesis of polymers that bear the chiral trans-1-phenylsulfonylamino-2-isoborneolsulfonylaminocyclohexane moiety was described by Yus and co-workers. Of the synthesised polymers, 9 was found to be the best promoter for the heterogeneous and homogenous catalytic enantioselective alkylation and arylation of ketones (54-99% yield, 38-90% ee). In this case the yield of recovered polymer was in the range of 75–90%. Work is still in progress in order to achieve recovery and reuse (Tetrahedron: Asymmetry2006, 17, 2054. DOI: 10.1016/j.tetasy.2006.07.038).