The asymmetric hetero-Diels-Alder (HDA) reaction is among the most powerful
available methodologies for the construction of optically active six-membered
heterocycles, with extensive synthetic applications in natural or unnatural
products with a wide range of biological activity. The DA reaction has high
regioselectivity and endo-stereoselectivity. This Highlight reviews recent
developments from 2005 to present (for comprehensive reviews on asymmetric DA
reaction see a) Angew. Chem. Int. Ed. 2002, 41, 1650.
DOI: 10.1002/1521-3773(20020517)41:10%3C1650::AID-ANIE1650%3E3.0.CO;2-B;
b) Angew. Chem. Int. Ed. 2002, 41, 1668. 4-Aminobutan-1-ol uses
DOI: 10.1002/1521-3773(20020517)41:10%3C1668::AID-ANIE1668%3E3.0.CO;2-Z;
c) Mini-Reviews in Organic Chemistry, 2004, 1, 41.
Link). (R)-1-(2-Pyridyl)ethylamine Purity Some of the examples presented here use
organocatalysis, which is a
rapidly growing research field.

|
|
| Kobayashi and co-workers (Tetrahedron Lett. 2005, 45, 1803. DOI: 10.1016/j.tetlet.2005.01.111) |
Dubernet and co-workers (Bioorg. Med. Chem. Lett. 2006, 16, 1172. DOI: 10.1016/j.bmcl.2005.11.100) |
|
|
| Burke and co-workers (J. Org. Chem. 2005, 70, 3757. PMID:24268253 DOI: 10.1021/jo050034v) |
|
Fig. 1 Recent examples using the HDA methodology.
1. aza-Diels-Alder Reactions
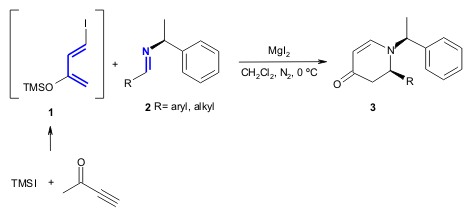
Timmons and co-workers reported the first aza-DA reaction using a new class
of iododiene 1 and chiral imines 2 (Tetrahedron 2005,
61, 11837.
DOI: 10.1016/j.tet.2005.10.018). The
methodology was used in the synthesis of dihydropyridones 3 (3:1 to 4.5:1 de,
61-85% yield).
Córdova and co-workers reported the first one-pot three-component direct
catalytic enantioselective aza-DA reaction. This organocatalysed reaction was
tested using different ketones 4 (40-90% yield, 94-99% ee), formaldehyde (5) and anilines
6 (20-70% yield, 96-99% ee) in the presence of (S)-proline. The
reaction occurs via a transition state in which the in situ-generated imine
attacks the si face of the proline-derived catalyst. The reaction is simple, can
be performed in wet solvents, and is environmentally friendly (Angew. Chem. Int.
Ed. 2005, 44, 4877.
DOI: 10.1002/anie.200500811).
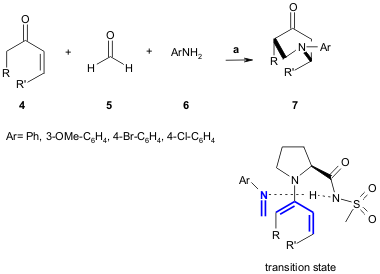
a) 4 (2 mmol, 2 equiv.), aqueous 5 (1 mmol), 6 (1.1 mmol), (S)-proline (30
mol%), DMSO, rt, 20-72 h.
2. oxo-Diels-Alder Reactions
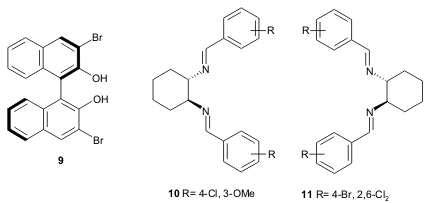
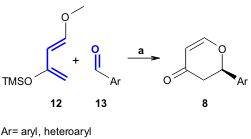
Ding and coworkers successfully developed a catalytic oxo-DA reaction for the
synthesis of 2,3-dihydro-4H-pyran-4-one derivatives 8 in excellent yields and
high enantioselectivity. The authors used chiral zinc catalysts containing
ligand 9, various diimine activators (the best results were obtained using
10
and 11), Danishefsky’s-type diene 12 and aromatic aldehydes
13 through a
combinatorial approach (Tetrahedron 2005, 61, 9465.
DOI: 10.1016/j.tet.2005.08.007).
a) 1. ligand/Et2Zn/diimine (10 mol%), toluene, -20°C ; 2. CF3CO2H
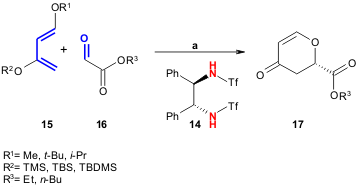
Using chiral lanthanide complexes with (R,R)-DPENTf (14) Tonoi and Mikami
report a catalytic oxo-DA reaction between Danishefsky’s-type diene
15 and glyoxylate 16 (23-87% yield, 23-86% ee). The reaction proceeds through double
hydrogen bonding using bis-triflylamide as chiral Brønsted acid catalyst (Tetrahedron
Lett. 2005, 46, 6355.
DOI: 10.1016/j.tetlet.2005.07.043).
a) 15 (0.12 mmol), 16 (0.1 mmol), 14 (10 mmol%),
toluene, -20 or -78ºC, 4 h.
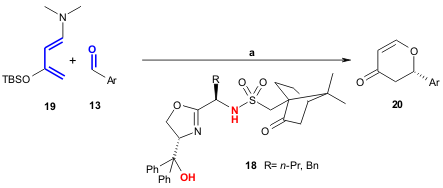
Rajaram and Sigman developed a new class of hydrogen bond catalysts 18 that
activate aldehydes 13 through hydrogen bonds. These rigid oxazolines
successfully (42-80% yield) promote highly enantioselective (71-92% ee) oxo-DA
reactions (Org. Lett. 2005, 7, 5473.
DOI: 10.1021/ol052300x). The authors found that both hydrogen
bond donors in the organocatalyst are necessary for effective catalysis.
a) 1. 18 (20 mol%), toluene; 2. CH2Cl2, CH3COCl, -78ºC.
Rawal and co-workers reported an oxo-DA organocatalysed reaction between
aminosiloxydiene 19 and a wide variety of unactivated aldehydes 21 with useful
yields (42-99% yield) and excellent enantioselectivities (84-99% ee) using
axially chiral diols 22 of the BAMOL family (J. Am. Chem. Soc. 2005,
127, 1336.
DOI: 10.1021/ja044076x). The authors show that the diols function in the same capacity as Lewis
acids, by activating the aldehyde carbonyl group through hydrogen bonding.
a) 1. 19 (0.5 mmol), 21 (1 mmol), 22 (0.1 mmol),
toluene, -40 or -80ºC; 2.
CH3COCl, CH2Cl2-toluene, -78ºC, 30 min.
3. Other Hetero Diels-Alder Reactions
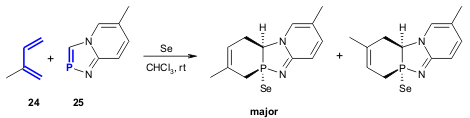
Kumawat and co-workers developed a regioselective (4:1 de, 50% yield) HDA
reaction between isoprene (24) and [1,4,2]diazaphospholo[4,5-a]pyridines
25 in the presence of selenium (Tetrahedron 2005, 61, 10521.
DOI: 10.1016/j.tet.2005.08.045). The weaker C=P π-bond, relatively to the C=C π-bond, lowers the activation energy needed for the
cyclisation and favours the HDA reaction.