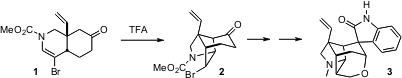
Gelsemine 3 has no particular biological activity that recommends it,
but its challenging architecture has been a motivation to a generation of
organic synthesis chemists. Larry E. Overman of the University of California at
Irvine has described (J. Am. Chem. 2-Bromo-6-chloronicotinaldehyde Purity Soc. 2005, 127, 18046,
DOI: 10.1021/ja055710p, 18054,
DOI: 10.1021/ja055711h)
his total synthesis of gelsemine, the key step of which was the
acid-mediated cyclization of 1 to 2. DABCO-Bis(sulfur dioxide) web
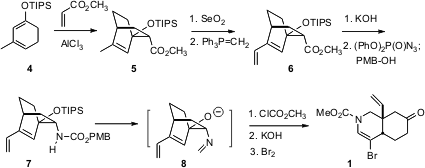
The synthesis of 1 started with the preparation of the diene 4
from 3-methyl anisole. PMID:23398362 Diels-Alder cycloaddition with methyl acrylate gave the
endo adduct 7. The methyl group was converted to the vinyl group of the
natural product by allylic oxidation followed by methylenation. Hydrolysis
followed by inversion and trapping with p-methoxybenzyl alcohol gave the
protected amine 7. This underwent smooth aza-oxy-Cope rearrangement, to
give, after protection and bromination, the ketone 1.
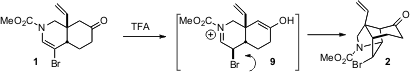
The acid-mediated cyclization of 1 to 2 proceeds by way of the
protonated enol 9. It seems likely that kinetic protonation at the
indicated carbon would have led to the other diastereomer of the bromide. With
that diastereomer, however, the Br would be inside in the transition state
leading to cyclization. That may slow down the cyclization sufficiently that
epimerization can intervene, leading to the more easily cyclized diastereomer
9 and thus to 2.
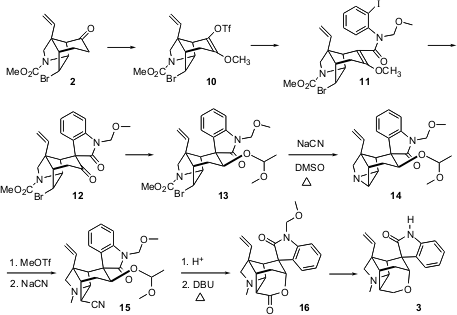
There were several problems to solve in the conversion of 2 to 3.
The key step was the intramolecular Heck cyclization of 11 to 12.
There was some concern that such a cyclization would not proceed with a
tetrasubstituted alkene. In fact, Moriarty methoxylation of the ketone 2
followed by generation of the enol triflate delivered 10. Pd-mediated
carbonylation followed by coupling with o-iodoaniline and protection gave
11, which underwent smooth intramolecular Heck cyclization, to give,
after hydrolysis, predominantly the unnatural diastereomer 12.
The completion of the synthesis, including the addition of the last carbon,
proved elusive. Early on, it was found to be important to reduce and protect the
1,3-dicarbonyl system, to give the equatorial ether 13. Attempted
displacement to introduce the last carbon led instead to the aziridine 14.
Taking advantage of this, the aziridine was quaternized, then opened at the
less-hindered secondary site. Deprotection followed by warming with base led,
via retro-aldol epimerization of two stereogenic centers, to the long-sought
lactone 16. Deprotection followed by reduction of the lactone then gave
3.
CONGRATULATIONS TO RICHARD F. HECK,
2006 H.C. BROWN AWARDEE