A variety of dolabellanes, some of which show substantial physiological
activity, have been isolated from natural sources. Cholesterol Price E. PMID:31085260 J. Corey of Harvard
University has introduced (J. Am. Chem. Soc. 127273-06-7 Chemical name 2005, 127,
13813.
DOI: 10.1021/ja055137+)
a
unfied approach to the dolabellanes, represented by isoedunol (3), based
on the designed rearrangement of the mesylate 1 to 2.
The key to this approach was the stereocontrolled construction of the
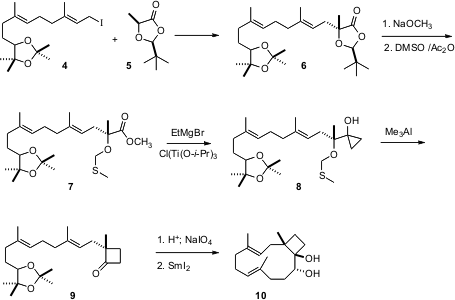
cyclobutane 1. The starting material was the racemic iodo acetonide 4
derived from farnesol. Alkylation of 5 using the Seebach protocol
followed by hydrolysis led the methylthiomethyl ether 7. The ester was
converted to the hydroxy cyclopropane 8 by the
Kulinkovic procedure. On
activation with Me3Al, 8 was smoothly carried on to the
enantiomerically-pure cyclobutanone 9. The ring expansion must not be
proceeding by full ionization, as carbocation formation would have led to the
racemic product. The aldehyde derived from 9 was cyclized with SmI2
to the trans diol 10.
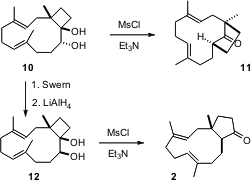
In medium ring derivatives such as 10, one substituent on a ring
carbon will be inside, and the other will be outside. The conformation of 10
is such that formation of the mesylate from the secondary alcohol led to
migration of the more substituted cyclobutane bond, delivering 11. It
follows that the conformation of the diol 12 will be flipped, to keep the
OH outside the ring. Formation of the mesylate from the secondary alcohol of
12 led cleanly to migration of the less substituted cyclobutane bond, to
give the desired cyclopentanone 2.
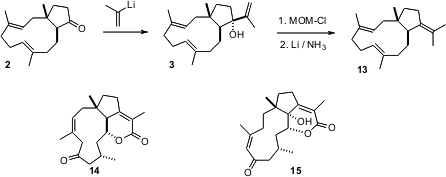
Addition of 2-propenyl lithium to the cyclopentanone 2 gave the
dolebellane isoedunol (3). Deoxygenation converted 3 to the
dolabellane β-araneosene (13). This strategy for the construction of the
dolabellanes may open a route for the preparation of the cytotoxic dolabellanes
clavulactone (14) and clavirolide (15).