The indole nucleus, known as a privileged platform, is present in a wide range of natural products and important synthetic molecules. Price of 623583-09-5 Many strategies have been developed for the catalytic asymmetric alkylation of indoles; however, only recently have enantioselective methods been reported.
Part I. Enantioselective Organocatalytic Indole Alkylation
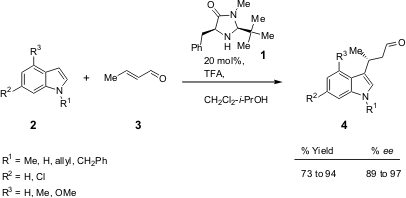
Austin and MacMillan (J. Am. Chem. PMID:24257686 Soc. 2002, 124, 1172. DOI: 10.1021/ja017255c)reported the enantioselective organocatalytic alkylation of the indole nucleus using the imidazolidinone catalyst 1 (Scheme 1).
Scheme 1 – Enantioselective alkylation of indole using 1 as organocatalyst.
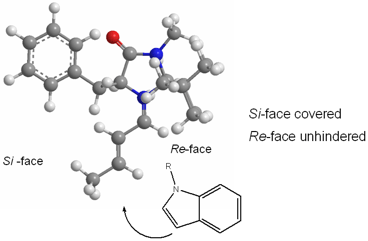
The catalyst design was based on kinetic studies and enantiocontrol criteria: control of iminium ion geometry ((E)-isomer), enantiofacial discrimination, and avoidance of nonbonding interactions between the olefin and the t-butyl group present in 1 (the Re-face is left free for the addition reaction – see Figure 1). This catalyst proved to be very effective for the enantioselective alkylation of several indoles (up to 97% ee and up to 94% yield), including indoles that contain electron-deficient groups such as 6-chloroindole. These halogenated indole adducts are important building blocks for drug synthesis, as they can be used in coupling processes. Buy355819-02-2
Figure 1 – Computational model of iminium ion of 1, showing freedom from steric hindrance.
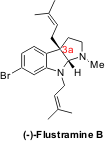
Recently, this concept has been extended to the synthesis of pyrroloindoline alkaloids (PNAS 2004, 101, 5482.DOI: 10.1073/pnas.0308177101). These alkaloids exhibit diverse biological activity and are structurally complex, making them challenging synthetic targets that attract the attention of many research groups. Austin and MacMillan developed a new catalytic enantioselective approach that allows the construction of the pyrroloindoline skeleton in one step, with stereocontrol at C(3a) (for example, in (-)-Flustramine B, Figure 2).
Figure 2 – (-)-Flustramine B, a representative example of pyrroloindoline alkaloids.
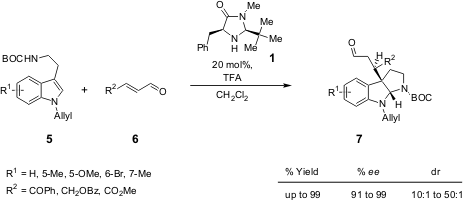
The authors’ strategy consisted of a cascade sequence (conjugate addition-cyclisation) to form the pyrroloindoline skeleton from tryptamines and simple α,β-unsaturated aldehydes (Scheme 2), using the chiral amine catalyst1. These authors call our attention to the fact that the solvent proved to be very important. While best results were obtained with CH2Cl2-H2O for reactions with acrolein, the use of dry CH2Cl2 resulted in higher enantioinduction with β-substituted α,β-unsaturated aldehydes. The versatility of this method has been demonstrated by the results obtained with diverse tryptamine derivatives 5 which were used successfully with unsaturated aldehydes 6 (high yield up to 99% and high enantioselectivity up to 99% ee).
Scheme 2 – Enantioselective construction of the pyrroloindoline system by conjugate addition-cyclisation process.
The Denhart group applied this method to the synthesis of homotryptamines in one-pot directly from indoles and acrolein with subsequent reductive amination (Tetrahedron Lett. 2004, 45, 3803.DOI: 10.1016/j.tetlet.2004.03.070). However, only indoles without strong electron-withdrawing groups and α,β-unsaturated aldehydes that possesses no α-substituents were tested.
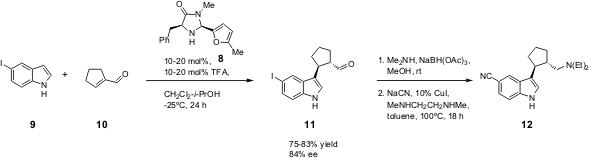
Recently, this group (Org. Lett. 2005, 7, 3437.DOI: 10.1021/ol051000c)has enlarged the scope of this method, by applying the MacMillan/Northrup catalyst 8 (J. Am. Chem. Soc. 2002, 124, 2458. DOI: 10.1021/ja017641u)to the synthesis of the highly potent Selective Serotonin Reuptake Inhibitor (SSRI) 12 (Scheme 3).
Scheme 3 – Synthesis of (SSRI) 12 via enantioselective alkylation of indole 9 catalysed by 8.
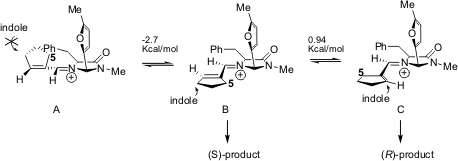
Catalyst 1 was not effective on alkylations with α-branched substrates, whilst catalyst 8 proved to be very effective at allowing the formation of the corresponding adducts with high enantiocontrol (up to 85% ee). The synthesis of 12 was performed with 5-iodoindole (9), since 5-cyanoindole only gave trace amounts of the desired product. In this case the (S)-product11 was isolated, instead of the (R)-product isolated by MacMillan. The authors give an explanation for the observed preference based on molecular modelling studies: the (E)-iminium ion A has the Si-face sterically blocked by the benzyl group so the indole cannot add to the cyclopentene; the trans-Z-iminiumB is preferred to the cis-Z-iminium C since the latter is 0.94 Kcal/mol higher in energy. The trans-Z-iminium B represents the more favourable conformation that avoids the steric interactions between C-5 and benzyl group (Scheme 4).
Scheme 4 – Proposed mechanism for the observed enantioselectivity of the indole alkylation.
The use of an organocatalyst derived from phenylalanine was developed as a new strategy in asymmetric synthesis, in particular in indole alkylation by lowering the LUMO of the olefin via the reversible formation of an iminium ion.