Aromatic and heteroaromatic rings are the heart of pharmaceutical design. Several useful methods for monocyclic and polycyclic aromatic ring construction have recently been reported.
Aaron L. PMID:32695810 Odom of Michigan State University has described (Org. Lett. 2004, 6, 2957. Formula of 2,5-Dimethoxy-4-formylphenylboronic acid DOI: 10.1021/ol0489088)a new approach to dialkyl pyrroles. Ti-catalyzed hydroamination of a 1,4-diyne such as 1 leads smoothly to 2. 220497-67-6 structure Similarly, Ti-catalyzed hydroamination of a 1,5-diyne such as 3 delivers 4. An inherent limitation of this approach is that it only allows substitution at the 2 and the 5 positions of the pyrrole.
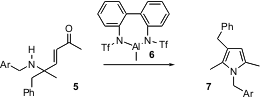
A more flexible approach to pyrroles has been developed (Tetrahedron Lett. 2004, 45, 9315. DOI: 10.1016/j.tetlet.2004.10.126)by Keiji Maruoka of Kyoto University. Rearrangement of 5 by the Al complex 6 leads, by preferential benzyl migration, to the trisubstituted pyrrole 7.
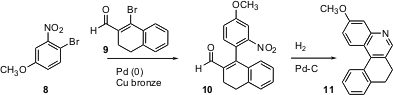
Martin Banwell of the Australian National University has devised (Org. Lett. 2004, 6, 2741.DOI: 10.1021/ol0490375)a Pd-mediated approach to fusedquinolines. Coupling of 8 with the bromo aldehyde 9 leads to 10, which on reduction cyclizes to 11.
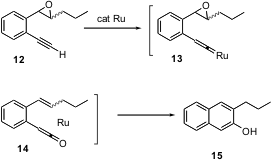
Substituted naphthalenes can be prepared by adding an aromatic ring onto an existing benzene derivative. Rai-Shung Liu of the National Tsing-Hua University, Taiwan, has found (J. Am. Chem. Soc. 2004, 126, 6895. DOI: 10.1021/ja049943c)that exposure of an epoxy alkyne such as 12 to a catalytic amount of TpRuPPh3(CH3CN)2PF6 leads to the β-naphthol15. The reaction is thought to be proceeding by initial rearrangement to the vinylidene complex 13. O-atom transfer converts 13 to the ketene14, which undergoes cycloaddition to the newly-liberated alkene to give 15. The facile conversion of 12 to the TpRu-vinylidene complex 13 will have many other applications in organic synthesis.