Applications to natural product synthesis probe the limits of any synthetic method, as situations arise that would never have been considered in the course of first developing the method. PMID:35567400 Recent syntheses of valienamine (6), agelastatin (9) and tonantzitlolone (15) pressed the limits of ring-closing metathesis. 1,2,3,4-Tetrahydroquinolin-5-ol Chemscene Tributyl(1-ethoxyethenyl)stannane site
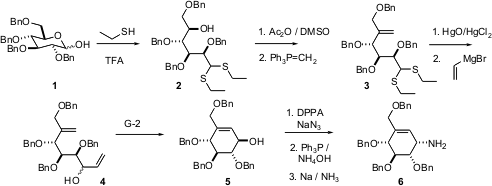
The efficient conversion of carbohydrates to highly-oxygenated, enantiomerically-pure carbocycles has long been a goal of organic synthesis. Heung Bae Jeon and Kwan Soo Kim of Yonsei University, Seoul, have optimized the conversion (J. Org. Chem. 2005, 70, 3299. DOI: 10.1021/jo047735x)of the commercial 2,3,4,6-tetra-O-benzyl-D-glucopyranose (1) to the alkene 3. Hydrolysis of the thioacetal 3 delivered an unstable aldehyde, that was reacted with vinyl magnesium bromide to give 4 as an inseparable 7:3 mixture of diastereomers. The highly-oxygenated diene 4 participated smoothly in the Grubbs cyclization, to give the easily-purified alcohol 5 as the major product. The alcohol 5 was carried on to the α-glucosidase inhibitor valienamine (6).
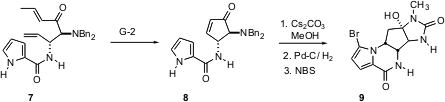
Highly aminated substrates also participate smoothly in the Grubbs cyclization. Franklin A. Davis of Temple University has reported (Org. Lett. 2005, 7, 621.DOI: 10.1021/ol047634l)the preparation of the enantiomerically-pure 1,2-diamine 7 using his elegant sulfinimine addition. Remarkably, the highly-aminated diene 7 participated smoothly in the Grubbs cyclization. The product8 was carried on to the novel antitumor agent (-)-agelastatin A (9), the central cyclopentane ring of which is unusual in having four aminated stereogenic centers.
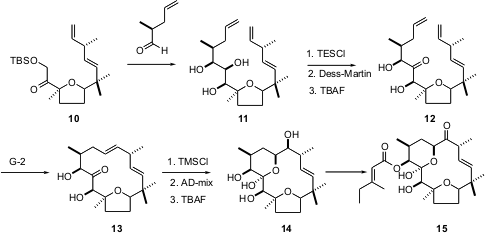
The vagaries that can afflict any complex natural product synthesis are well illustrated by the route to the cytotoxic diterpene tonantzitlolone (15) developed (Org. Lett. 2005, 7, 479.DOI: 10.1021/ol047559e)by Andreas Kirschning of the Universität Hannover. Their first approach, based on ring-closing metathesis to form the single alkene of 15, had to be abandoned, because metathesis failed, probably because the product alkene was too congested. This led them to the approach illustrated, the expectation being that if the other alkene was too congested to be formed by ring-closing metathesis, it would be too congested to interfere with ring-closing metathesis. Aldol addition of 10 proceeded with remarkably high diastereoselectivity, leading to 11. Ring-closing metathesis of the keto diol went well, setting the stage for the preparation of 15. The synthetic material so prepared turned out to be the enantiomer of the natural product.