Arrays of Stereogenic Centers
Single enantiomer synthesis is of increasing importance in pharmaceutical
production. It is essential that practical and scalable procedures be developed
for controlling the absolute configuration of new stereogenic centers as they
are formed. Tris(pyrazol-1-yl)methane Order In the previous column, recent advances
for the preparation of single stereogenic centers were covered. The construction
of more extended arrays of stereogenic centers, which is also is important, is
covered here. (2-Hydroxyethyl)trimethylsilane manufacturer
One approach is to use enantioselective methods to establish a first stereogenic
center, then use that center to control the relative configuration of additional
centers as they are formed. PMID:35954127 Patrick Walsh of the University of Pennsylvania has
found (J. Am. Chem. Soc. 2004, 126, 13608.
DOI: 10.1021/ja046750g)
that
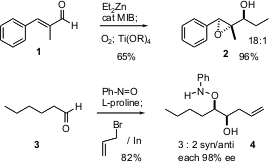
addition of a dialkyl zinc reagent to an aldehyde such as 1, using the
high e.e. Nugent procedure, gives an intermediate that on exposure to molecular
oxygen gives the epoxy alcohol 2 with high diastereomeric control. In a
complementary approach, Guofu Zhong of the Scripps Institute, La Jolla has shown
(Chem. Commun. 2004, 606.
DOI: 10.1039/b314356b)
that
enantioselective aminoxylation of an aldehyde such as 3 can be followed
in the same pot by the addition of an organometallic reagent, to give the
monoprotected diol 4 in high enantiomeric excess. While the
diastereomeric control is low in this case, one would expect this to improve if
a bridging Lewis acid were included.
Enantioselective aldol reactions also can be used to create arrays of
stereogenic centers. Two elegant α-amino anion approaches have recently been
published. Fujie Tanaka and Carlos F. Barbas III of the Scripps Institute, La
Jolla, have shown (Org. Lett. 2004, 6, 3541.
DOI: 10.1021/ol0485417)
that
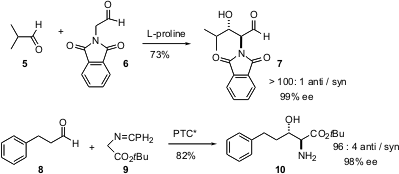
L-proline catalyzes the addition of the aldehyde 6 to other aldehydes
with high enantio- and diastereocontrol. Keiji Maruoka of Kyoto University has
developed (J. Am. Chem. Soc.
2004, 126, 9685.
DOI: 10.1021/ja048865q)
a chiral phase transfer catalyst that mediates the addition of the ester 9 to
aldehydes, again with high enantio- and diastereocontrol.
Michael addition can also be used to establish arrays of stereogenic centers.
Hiyoshuzi Kotsuki of Kochi University has shown (J. Am. Chem. Soc.
2004,
126, 9558.
DOI: 10.1021/ja046871g)
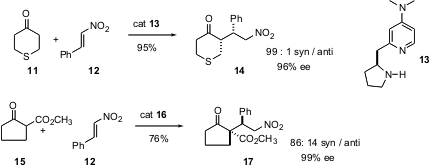
that the chiral DMAP derivative
13 mediates the addition of
cyclic ketones such as 11 to
nitrostyrene 12 with high enantio- and diastereocontrol. Acyclic
aldehydes also add with high stereocontrol. Li Deng of Brandeis University has
developed (Angew. Chem. Int. Ed. 2005, 44, 105.
DOI: 10.1002/anie.200461923)
a
quinine-based catalyst
16 that directs the addition of 12 to a single face of the cyclic
β-ketoester
15, establishing adjacent ternary and quaternary centers. For the
conversion of the nitro group to a nitrile without epimerization, see Angew.
Chem. Int. Ed.
2005,
44, 612,
DOI: 10.1002/anie.200461879).
The construction of more extended arrays is also possible. Justin Du Bois of
Stanford University has reported (Angew. Chem. Int. Ed. 2004,
43, 4349.
DOI: 10.1002/anie.200460791)
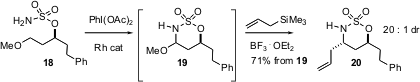
diastereoselective remote functionalization using C-H insertion of a sulfamate
such as 18 to give the oxathiazinane 19, which on reaction with an
allyl silane gives the alkylated product 20 with high diastereocontrol.
Note that the oxygen of 20 is activated as a leaving group.