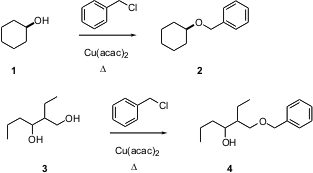
Benzyl ethers are among the most commonly used protecting groups for alcohols. PMID:23554582 Usually, these are prepared using an excess of NaH and benzyl bromide. Buy1-(Aminomethyl)cyclopentanol Okan Sirkecioglu of Istanbul Technical University has found (Tetrahedron Lett. 2003, 44, 8483.DOI: 10.1016/j.tetlet.2003.09.106)that heating a primary or secondary alcohol neat with benzyl chloride and a catalytic amount of Cu(acac)2 smoothly yields the benzyl ether, with evolution of HCl. 8-Bromoquinazoline-2,4-diol structure The reaction can also be run in solvent THF, but it proceeds more slowly. Note that primary alcohols react more quickly than secondary alcohols.
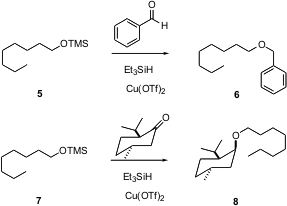
Usually, one would consider the conversion of an aldehyde or a ketone to the ether to be a two-step process. There are, however, catalysts that will effect this conversion in a single step, as exemplified by the work of Shang-Cheng Hung of Academia Sinica in Taipei (Tetrahedron Lett. 2003, 44, 7837.DOI: 10.1016/j.tetlet.2003.08.085). Although one might choose to use this as another alternative for preparing benzyl ethers, note that it also offers an efficient procedure for preparing unsymmetrical dialkyl ethers, which can sometimes be a difficult task. The diastereoselectivity of the process is particularly impressive.
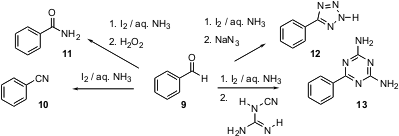
Several years ago, Jim-Min Fang of National Taiwan University reported that an aldehyde 9 was smoothly converted into the corresponding nitrile 10 byiodine in aqueous ammonia. He has now observed (J. Org. Chem. 2003, 68, 1158.DOI: 10.1021/jo026407z)that the intermediate nitrile can be carried on in situ to the amide11, the tetrazole 12, or the triazine 13.