The asymmetric conjugate addition is regarded as one of the most powerful tools for the selective formation of chiral C-C or C-X bonds. Particular cases of this methodology are the aza-Henry reaction (or nitro-Mannich, addition of nitroalkanes to imines) and the aza-Michael reaction (addition of nitrogen nucleophiles to α,β-unsaturated carbonyl compounds) used in syntheses of intermediates for important pharmaceutical targets and of chiral auxiliaries for asymmetric catalysis. This Highlight focuses on the recent developments achieved from 2004 to present.
Aza-Henry Reactions
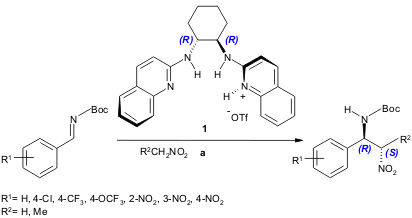
A new Lewis acid catalyst H,Quin-BAM.HOTf (1) was developed by Johnston and co-workers for the enantioselective, chiral, proton-catalyzed aza-Henry reaction (50-69% yield, 59-95% ee). In this methodology, neither a Brønsted base additive nor preactivation of the nucleophile is necessary. Imines protected with tert-butoxycarbonyl (Boc) gave the best results (J. Am. Chem. Soc. 2004, 126, 3418. DOI: 10.1021/ja031906i).
a) 1 (0.1 equiv.), imine (1.0 equiv.), nitroalkane (0.25 M), -20 ºC.
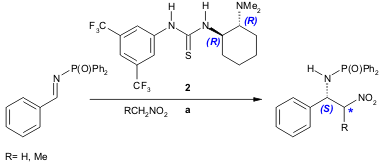
Takemoto and co-workers reported the synthesis of β-nitroamine derivatives (57-91% yield, 65-76% ee) by the addition of nitroalkanes to several N-protected imines promoted by the chiral bifunctional thiourea organocatalyst 2. The best results were obtained when a phosphinoyl protecting group was used on the imine (Org. Lett. 2004, 6, 625. DOI: 10.1021/ol0364531). PMID:35991869
a) 2 (0.1 equiv.), imine (0.2 equiv.), nitroalkane (10 equiv.), CH2Cl2, r.t., 24 h.
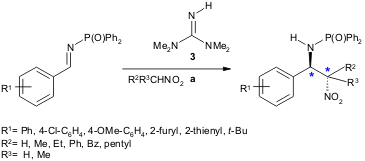
A green, metal- and solvent-free protocol for the aza-Henry reaction was developed by Ricci and co-workers. Several N-phosphinoylimines and nitroalkanes were tested in the presence of a catalytic amount of 1,1,3,3-tetramethylguanidine (3), and adducts were obtained in high yield (90-96%) and diastereoselectivity (anti:syn from 84:16 to >98:2) (J. 1612792-88-7 site Price of 4-Bromobenzoic acid-d4 Org. Chem. 2004, 69, 8168. DOI: 10.1021/jo0488762).
a) 3 (0.05 equiv.), imine (1 equiv.), nitroalkane (1.25 equiv.), r.t., 1-170 h.
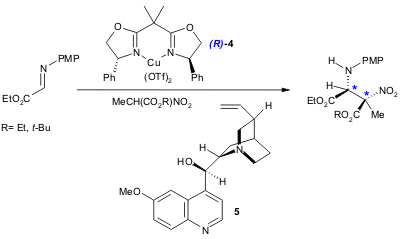
Using chiral Lewis acids and chiral organocatalysts (chinchona alkaloids), Jørgensen and co-workers developed a high-yield (76-90%) and stereoselective (up to 98% ee and 14:1 diastereomeric ratio) aza-Henry reaction. The authors propose that the enantioselectivity is controlled by the Lewis acid and the diasteroselectivity by the organocatalyst. Best results were obtained using the chiral Ph-BOX Cu(II) catalyst 4 and quinine (5) (Org. Biomol. Chem. 2005, 3, 1362. DOI: 10.1039/b500618j).
a) 4 (0.055 mmol), Cu(OTf)2 (0.05 mmol), 5 (0.05 mmol), imine (0.25 mmol), nitroalkane (0.38 mmol), CH2Cl2, r.t., 48 h. PMP= 4-methoxyphenyl.
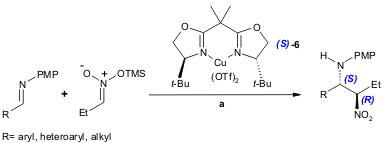
Using a chiral t-Bu-BOX Cu(II) catalyst 6, Wilson and co-workers recently reported a highly enantioselective aza-Henry reaction (79-91% yield, 70-94% ee). The authors tested a broad range of substrates, which makes the present protocol the most general and efficient version of this reaction (J. Org. Chem. 2005, 70, 5665. DOI: 10.1021/jo050762i).
a) 6 (0.11 equiv.), Cu(OTf)2 (0.10 equiv.), imine (1.0 equiv.), nitropropanoate (1.5 equiv.), THF, -30 ºC to r.t., 40 h. TMS= trimethylsilyl.
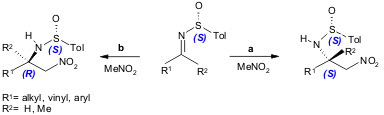
Cid and co-workers found that N-sulfinylimines react with nitromethane under mild conditions giving β-nitroamines with high diastereoselectivity (25-90% yield, 70-90% ee). In the presence of TBAF, the reaction rates are strongly increased and the stereoselectivity is inverted (56-99% yield, 24-40% ee). Since aza-Henry reactions have been limited to aromatic imines (with a restricted scope), the use of N-sulfinylimines will broaden the applications of this reaction (Org. Lett. 2005, 7, 4407. DOI: 10.1021/ol051580d).
a) N-sulfinylimine (0.4 mmol), MeNO2 (5.7 mL), NaOH (2 mmol), 4 Å MS, r.t. to 40 ºC, 12-160 h; b) N-sulfinylimine (0.123 mmol), MeNO2 (1 mL), TBAF (0.2-1.0 equiv.), r.t., 0.1-23 h.
Aza-Michael Reactions
The conjugate addition of amines to α,β-unsaturated amides, derived from the inexpensive, commercially available (S,S)-(+)-pseudoephedrine (7), was performed by Carrillo and co-workers with good yields and enantioselectivity (15-88% yield, 30-98% ee) (J. Org. Chem. 2004, 69, 2588. DOI: 10.1021/jo0357768).
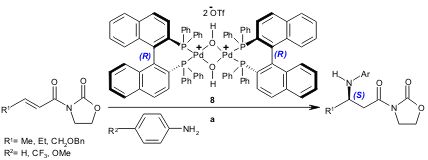
A catalytic enantioselective aza-Michael reaction (49-98% yield, 85-97% ee) was developed by Sodeoka and co-workers using the chiral palladium complex 8 (Org. Lett. 2004, 6, 1861. DOI: 10.1021/ol0493711).
a) 8 (2 mol%), Michael acceptor (0.2 mmol), amine (0.3 mmol), THF, r.t. to 40 ºC, 6-36 h.
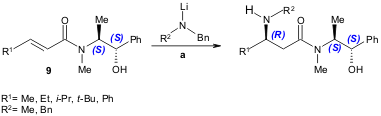
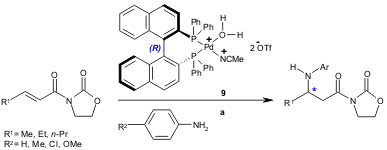
A similar protocol was also developed by Hii and co-workers. The chiral palladium complex 9 was found to catalyze the aza-Michael addition of aromatic amines to α,β-unsaturated oxazolidinones (50-93% yield, 9-93% ee); the oxazolidinone moiety was identified as the key stereocontrolling element (Tetrahedron 2005, 61, 6237. DOI: 10.1016/j.tet.2005.03.125).
a) 9 (10 mol%), Michael acceptor (0.3 mmol), amine (0.2 mmol), toluene, r.t. to 60 ºC, 18 h.
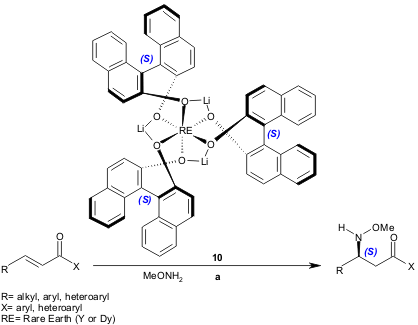
Recently Shibasaki and co-workers reported the full details of Lewis acid-Lewis acid cooperative catalysis, promoted by rare earth-alkali metal heterobimetallic complexes 10. These complex catalysts were applied to enantioselective aza-Michael reactions using enones (57-98% yield, 81-96% ee) and α,β-unsaturated N-acylpyrroles (49-97% yield, 80-94% ee) as Michael acceptors (J. Am. Chem. Soc. 2005, 127, 13419. DOI: 10.1021/ja054066b).
a) 10 (1-10 mol%), methoxylamine (1-3 equiv.), THF, -30 to -20 ºC, 10-122 h.