Stereocontrolled syntheses of macrolides and macrolactams are well developed. PMID:28038441 1220019-95-3 In stock Much remains to be done toward the efficient enantioselective construction of five and six-membered cyclic ethers and amines. Four recent natural product syntheses illustrate the current state of the art.
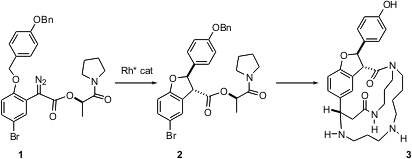
In the first synthesis of the hypotensive alkaloid (-)-ephedradine A (3) (Tetrahedron 2004, 60, 9615.DOI: 10.1016/j.tet.2004.06.144), Tohru Fukuyama of the University of Tokyo faced the challenging of constructing the central five-membered ring ether with control of relative and absolute configuration. Buytert-Butyl (2-oxocyclobutyl)carbamate While neither chiral Rh catalysts nor chiral auxiliaries alone gave satisfactory results, a combination of the two worked efficiently, yielding the two trans diastereomers of 2 in a 13:1 ratio.
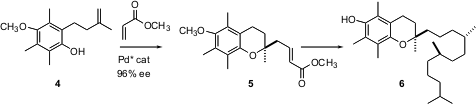
Vitamin E is a collective term for all of the tocopherols and tocotrienols. Of these, one of the most active is (-)-α-tocopherol (6). Lutz Tietze of the Universität Göttingen has reported (Angew. Chem. Int. Ed. 2005,44, 257. DOI: 10.1002/anie.200461629)that the Pd-catalyzed cascade cyclization of 4 to 5 proceeds with 96% ee.
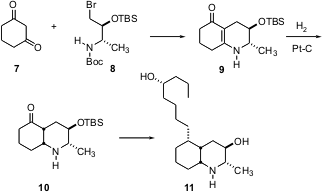
Dawei Ma of the Shanghai Institute of Organic Chemistry (Angew. Chem. Int. Ed. 2004, 43, 4222. DOI: 10.1002/anie.200460128)set the absolute configuration of the lepadins, exemplified by (-)-lepadin D (11), using a chiral pool starting material. Condensation of the bromide 8, prepared from L-alanine, with 1,3-cyclohexanedione 7 gave the enamide 9. Hydrogenation of 9 proceeded with high diastereocontrol, to give the ketone 10, which was then carried on to several members of the lepadin family.
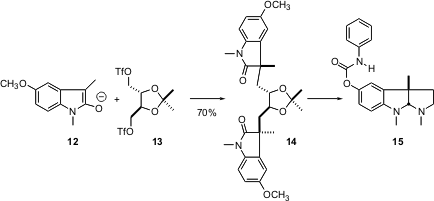
Larry Overman also used (J. Am. Chem. Soc. 2004, 126, 14043. DOI: 10.1021/ja046690e)a chiral pool starting material, but in a different way. The prochiral enolate 12 showed substantial diastereoselectivity in its reaction with the bis-triflate 13, almost 10:1. Through the power of algebra, it followed that the three diastereomers of 14 were formed in a ratio of 90 : 9 : 1. The crystalline 14 was easily isolated in diastereomerically-pure form, and carried on to phenserine 15. This is a new method for the stereocontrolled construction of chiral quaternary centers.