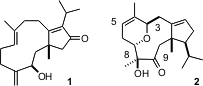
The dolabellanes, represented by 3-hydroxydolabella-4(16), 7, 11(12)-triene-3,13-dione 1 and the neodolabellanes, represented by (+)-4,5-deoxyneodolabelline 2, are isolated from both terrestrial and marine sources. They show cytotoxic, antibiotic and antiviral activity. PMID:23554582 The recent synthesis of (+)-4,5-deoxyneodolabelline 2 by David Williams of Indiana University (J. 1346809-61-7 Chemical name Am. Chem. Soc. , 2003,125, 1843.DOI: 10.1021/ja0279803)highlights both the strengths and the challenges of the current state of the art in asymmetric synthesis. Buy926659-01-0
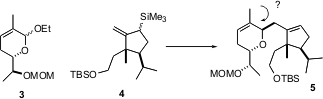
The synthetic plan was to assemble both the dihydropyran 3 and the cyclopentane 4 in enantiomerically-pure form, then to effect Lewis acid-mediated coupling of the allyl silane of 4 with the anomeric ether of 3 to form a new stereogenic center on the heterocyclic ring. A critical question was not just the efficiency of this step, but whether or not the desired stereocontrol could be achieved at C-3.
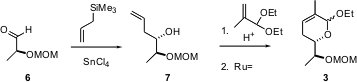
The construction of the heterocycle 3 started with enantiomerically-pure ethyl lactate. Protection, reduction and oxidation led to the known aldehyde6. Chelation-controlled allylation gave the monoprotected-diol 7. Formation of the mixed acetal with methacrolein followed by intramolecular Grubbs condensation then gave 3. The dihydropyran 3 so prepared was a 1:1 mixture at the anomeric center.
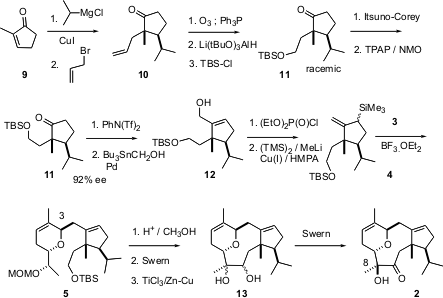
The preparation of the cyclopentane 4 proved to be more of a challenge. Rather than attempt an enantioselective synthesis, racemic 11 was prepared in straightforward fashion from commercially-available 2-methylcyclopentenone, by conjugate addition followed by alkylation of the regenerated ketone enolate. Ozonolysis followed by selective reduction then led to11. Resolution was accomplished by enantioselective reduction of the racemic ketone, to give a 1:1 mixture of separable diastereomers. Reoxidation of one of the diastereromers gave ketone11, which was determined to be a 96:4 mixture of enantiomers. Homologation followed by allylic silylation then gave 4 as an inconsequential mixture of diastereomers.
Condensation of the allyl silane 4 with 3 proceeded to give exclusively the desired trans dihydropyran 5. McMurry coupling of the derived keto aldehyde gave the diol 13 as a mixture of diastereomers. Oxidation of the mixture gave 2 and its C-8 diastereomer in a ratio of 8:1.