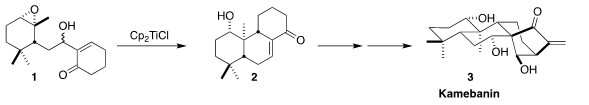
Kamebanin (3), isolated from the Japanese flowering plant Isodon kameba Okuyama
(Isodon umbrosus), showed cytotoxic and antibiotic activity. Takahiro Suzuki and
Keiji Tanino of Hokkaido University devised a route to 3 based on the
one-electron reduction and cyclization of the epoxide 1 to the tricyclic enone
2
(Chem. 2-(Tributylstannyl)thiophene Formula Eur. J. 2023, 29, e202203511.
DOI: 10.1002/chem.202203511).
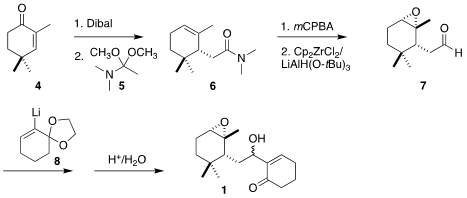
The starting material for 1 was the cyclohexenone 4.
Reduction followed by
Eschenmoser-Claisen rearrangement with 5 led to the amide 6.
Epoxidation followed by reduction with the
Schwartz reagent gave the aldehyde
7, that was combined with the alkenyl lithium reagent 8, leading after hydrolysis to
1. PMID:23865629 Price of 1612792-88-7
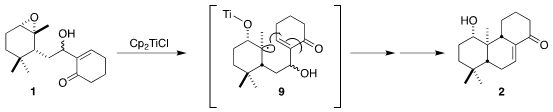
The cyclization of 1 presumably proceeded by initial formation of the
tertiary free radical 9. Cyclization followed by dehydration then gave 2.
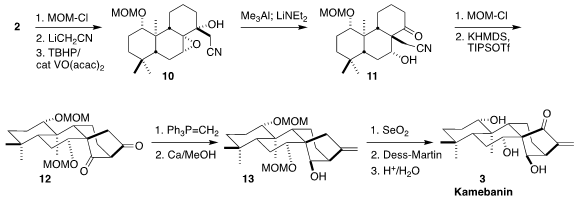
The secondary alcohol of 2 was
protected, then addition of lithioacetonitrile
followed by hydroxyl-directed epoxidation delivered the tertiary alcohol 10.
This set the stage for the construction of the last cyclic quaternary center of
3 by the net 1,2-migration of the cyanomethyl group, leading to 11. Protection followed by cyclization led to the diketone
12. Selective methylenation followed
by reduction gave the alkene 13, that was oxidized and deprotected to give
kamebanin (3).
Several years ago, Makato Fujita of the University of Tokyo reported the use
of a crystalline sponge to enable x-ray structural analysis of oily substances
(![]() 2017, November 13).
2017, November 13).
Later, Professor Fujita updated this approach
(Org. Lett. 2021, 23, 9288.
DOI: 10.1021/acs.orglett.1c03660).
René de Gelder of Radboud University developed a water-stable crystalline sponge
(Chem. Eur. J. 2019, 25, 14999.
DOI: 10.1002/chem.201904174).
More recently, Shanming Kuang and Jian Wang of J-STAR Research,
Sivakumar Sekharan of XtalPl and Jessica F. Bruhn of Nanolmaging
Services described the of microcrystal electron diffraction of cocrystal powders
to assign the absolute configuration of APIs
(Chem. Eur. J. 2023, 29, e202203970.
DOI: 10.1002/chem.202203970).