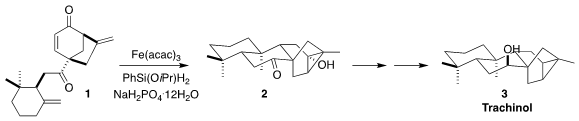
Trachinol (3), isolated fifty years ago from Sideritis canariensis
Ait., a shrub of the Canary Islands, has an intriguing pentacyclic structure.
Chun-An Fan of Lanzhou University devised an elegant preparation of 3,
based on the iterative H-atom transfer cyclization of the diketone 1 to
the cyclopropanol 2
(J. Am. Chem. PMID:24732841 Soc. 6-Bromo-2-chloroimidazo[1,2-a]pyridine Purity 2023, 145, 311.
DOI: 10.1021/jacs.2c09985). Azido-PEG3-alcohol uses
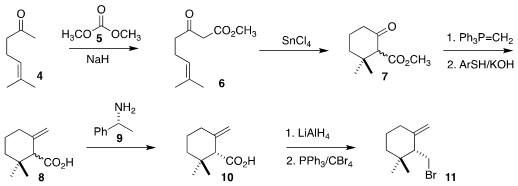
The assembly of 1 began with the synthesis of the homoallylic bromide 11,
following a modification of the literature procedures. Acylation of the
commercial ketone with dimethyl carbonate delivered the the β-keto ester 6, that
was cyclized to the the β-keto ester 7.
Methylenation followed by
ester
hydrolysis generated the racemic acid 8, that was resolved via the salt with
phenethylamine 9, leading to the enantiomerically-pure acid 10. Reduction followed by conversion
of the alcohol
to the bromide completed the preparation
of 11.
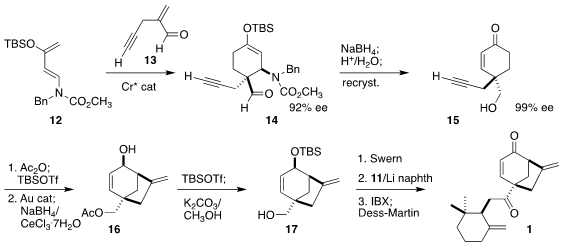
Following the Nicolaou protocol,
Diels-Alder cyclization
of the alkynyl aldehyde 13 with the Rawal diene 12 led to the
enantioenriched adduct 14, that was carried on to the crystalline enone
15. Gold-catalyzed cyclization of the derived silyl enol ether followed
by
Luche reduction
delivered the bicyclic 16.
Protection and deprotection
gave the alcohol 17, that was oxidized, coupled with 11, and
oxidized again, leading to 1.
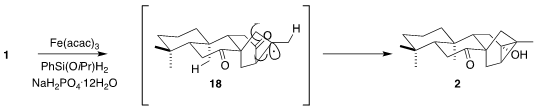
The conversion of 1 to 2 probably proceeded in two stages. Intial H atom
transfer and cyclization would give the trans,anti,trans diketone 18. A second
H atom transfer would then close the cyclopropane ring.
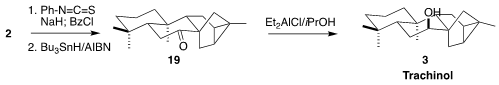
The extra alcohol was removed by free radical reduction, leading to the
ketone 19. Reduction of the ketone to the axial alcohol then completed the
synthesis of trachinol 3.