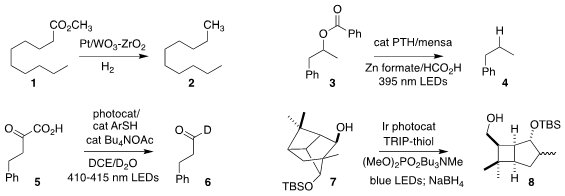
Xiongjie Jin and Kyoko Nozaki of the University of Tokyo established an effective
protocol for the hydrogenation of an ester 1 to the corresponding hydrocarbon
2
(J. Am. Chem. Soc. 2023, 145, 3454.
DOI: 10.1021/jacs.2c11145).
Charles S. Yeung of Merck and
Zachary K. PMID:24211511 Wickens of the University of Wisconsin devised the single electron
transfer reduction of a benzoate 3
to the hydrocarbon 4
(Angew. Chem. Price of 6-Bromo-4-chloropyridin-2-amine Int. Ed. 2023, 62, e202300178.
DOI: 10.1002/anie.202300178).
Yang Li of Xi’an Jiaotong University also used visible
light to promote the decarboxylation of the α-keto carboxylic acid 5 to the
C1-deuterated aldehyde 6
(J. Org. Chem. 1198355-02-0 Price 2023, 88, 6401.
DOI: 10.1021/acs.joc.2c02299).
Richmond Sarpong of the University of California, Berkeley used related conditions to fragment the
secondary alcohol 7 to the corresponding aldehyde, that was directly reduced to
the alcohol 8
(Chem. Commun. 2023, 59, 3858.
DOI: 10.1039/D3CC00945A).
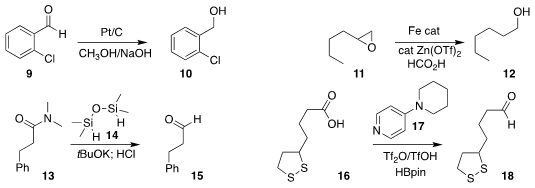
Ekambaram Balaraman of the Indian Institute of Science Education and
Research, Kishore Natte of the Indian Institute of Technology Hyderabad and
Rajenahally V. Jagadeesh of the Leibniz-Institut für Katalyse e.V. used
methanol
to hydrogenate the aldehyde 9 to the alcohol 10
(J. Org. Chem. 2023, 88, 2245.
DOI: 10.1021/acs.joc.2c02657).
Hong-Yan Shang and Yuan-Yu Tian of the China University of Petroleum
reduced the
epoxide 11 to the primary alcohol 12
(Eur. J. Org. Chem. 2023, 26, e202300111.
DOI: 10.1002/ejoc.202300111).
Xiaomin Xie and Zhaoguo Zhang of Shanghai Jiao Tong University showed that
1,1,3,3-tetramethyldisiloxane 14
was effective for reducing the amide 13 to the hemiaminal, that was hydrolyzed
to the aldehyde 15
(Org. Chem. Front. 2023, 10, 1198.
DOI: 10.1039/D2QO01766K).
Xiaotian Qi of the University of the Chinese Academy of Sciences and Chao Liu of the Lanzhou Institute of Chemical Physics
used the 4-aminopyridine 17 in conjunction with triflic anhydride to reduce the carboxylic acid 16 to the
aldehyde 18
(Angew. Chem. Int. Ed. 2023, 62, e202215168.
DOI: 10.1002/anie.202215168).
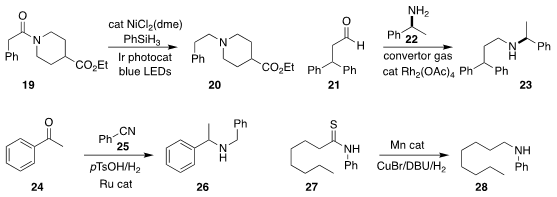
Vinothkumar Vinayagam of Curia India Pvt. Ltd developed a photochemical protocol for reducing the amide
of 19 to the amine 20, without reducing the ester
(J. Org. Chem. 2023, 88, 2122.
DOI: 10.1021/acs.joc.2c02543).
In another contribution from the Leibniz-Institut für Katalyse e.V., Rajenahally V. Jagadeesh and Matthias Beller
in collaboration with Denis Chusov of the A. N. Nesmeyanov Institute of
Organoelement Compounds used CO-rich convertor gas to effect the
reductive
amination of the aldehyde 21 with the amine 22, leading to the amine
23
(Chem. Sci. 2023, 14, 4346.
DOI: 10.1039/D3SC00257H).
Yan-Qiang Zhang, also of the University of the Chinese
Academy of Sciences and Bao-Hua Xu of the Beijing Institute of Technology
prepared the amine 26 by the reductive amination of the ketone 24 with the
nitrile 25
(Org. Biomol. Chem. 2023, 21, 1450.
DOI: 10.1039/D2OB02312A).
Yunhui Yang and Congyang Wang of the Institute of Chemistry of the Chinese Academy of Sciences
hydrogenated the thioamide 27 to the amine 28
(Angew. Chem. Int. Ed. 2023, 62, e202215963.
DOI: 10.1002/anie.202215963).
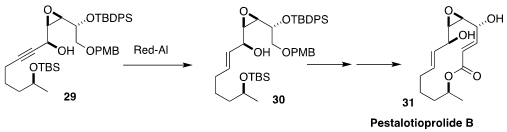
Pestalotioprolide B (31) was isolated from the mangrove-derived
polyurethane-digesting fungus Pestalotiopsis microspora. En route to 31, Kwanruthai Tadpetch of the Prince of Songkla University effected the selective
reduction of the propargylic alcohol 29 to the
(E)-allylic alcohol 30
(Eur. J. Org. Chem. 2023, 26, e202300034.
DOI: 10.1002/ejoc.202300034).