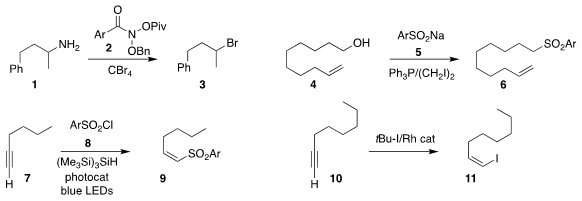
Christopher B. 2-Aminoacetamide uses Kelly of Janssen Discovery Process, Osvaldo Gutierrez of Texas
A&M and Mark D. Levin of the University of Chicago used the N-acyloxy
N-alkoxy amide 2 to convert the primary amine 1 to the corresonding
bromide 3
(J. Am. Chem. 1198605-51-4 manufacturer Soc. PMID:22664133 2023, 145, 17.
DOI: 10.1021/jacs.2c11453).
Xu Yao of the University of South China and
Jin-Hong Lin and Ji-Chang Xiao of the Shanghai Institute of Organic Chemistry prepared the
sulfone 6
by coupling the primary alcohol 4 with the arenesulfinate 5
(J. Org. Chem. 2023, 88, 4818.
DOI: 10.1021/acs.joc.2c03085).
Shuanglin Qu of Hunan University and Bo Zhang of China Pharmaceutical University added the
arenesulfonyl chloride 8 to the alkyne 7, leading to the
(Z)-unsaturated sulfone 9
(ACS Catal. 2023, 13, 6983.
DOI: 10.1021/acscatal.3c01529).
Bill Morandi of ETH Zürich used a Rh catalyst to convert the alkyne 10 to the
(Z)-alkenyl iodide 11
(Angew. Chem. Int. Ed. 2023, 62, e202214071.
DOI: 10.1002/anie.202214071).
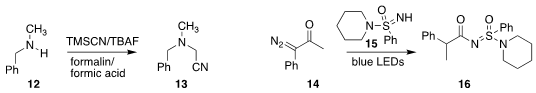
Do Hyun Ryu of Sungkyunkwan University and Hui Jin and Lixin Zhang of the
Shenyang University of Chemical Technology developed conditions for preparing
the cyanomethyl amine 13 from the secondary amine 12
(Adv. Synth. Catal. 2023, 365, 2.
DOI: 10.1002/adsc.202200767).
Carsten Bolm of RWTH Aachen University used visible light to promote
the Wolff rearrangement of the diazo ketone 14, leading to a ketene that
acylated the sulfonimidamide 15, to give 16
(Adv. Synth. Catal. 2023, 365, 31.
DOI: 10.1002/adsc.202201274).
Alessandro Palmieri of the University of Camerino converted the
readily-prepared nitro ketone 17 into the
diene 18
(Adv. Synth. Catal. 2023, 365, 13.
DOI: 10.1002/adsc.202201151).
Sebastien Stecko of the Institute of Organic Chemistry of the Polish
Academy of Sciences showed that
Wacker oxidation of the allylic amine 19 proceeded with high regioselectivity to give the
ketone
20
(Org. Biomol. Chem. 2023, 21, 115.
DOI: 10.1039/D2OB01843H).
Similar results have been achieved with allylic ethers
(![]() 2020, November 23).
2020, November 23).
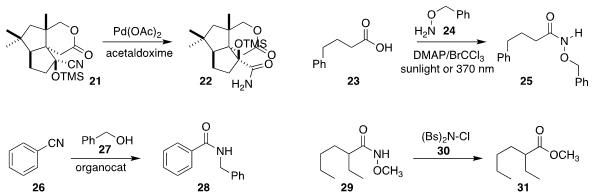
In the course of a synthesis of asperaculin A, Hisanaka Ito of the Tokyo
University of Pharmacy and Life Sciences used a Pd catalyst to convert the very
hindered nitrile 21 to the amide 22
(Org. Lett. 2023, 25, 4510.
DOI: 10.1021/acs.orglett.3c01530).
Christoforos G. Kokotos and George Kokotos of the National and Kapodistrian University of Athens
acylated the protected hydroxylamine 24 with the acid 23, leading to the
protected hydroxamic acid 25
(Eur. J. Org. Chem. 2023, 26, e202300046,
DOI: 10.1002/ejoc.202300046;
e202300008, DOI: 10.1002/ejoc.202300008).
Wen Hu of of Chongqing University used a 2,2′-bipyridinium salt to promote the
Ritter coupling of the alcohol 27 with the nitrile 26, to give the amide 28
(J. Org. Chem. 2023, 88, 4066.
DOI: 10.1021/acs.joc.2c02239).
Hemchandra Chaudhari of the Institute of Chemical Technology used N-chloro-N-(phenylsulfonylbenzenesulfonamide
30 to convert the N-alkoxy amide 29 to the
ester 31
(Tetrahedron Lett. 2023, 123, 154539.
DOI: 10.1016/j.tetlet.2023.154539).
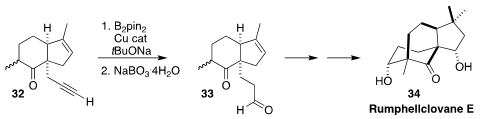
Rumphellclovane E (34) was isolated from the gorgonian coral Rumphella
antipathies. En route to 34, Shota Nagasawa and Yoshiharu Iwabuchi of Tohoku
University effected the selective conversion of the alkyne 32 to the
aldehyde
33
(Org. Lett. 2022, 24, 7572.
DOI: 10.1021/acs.orglett.2c02960).