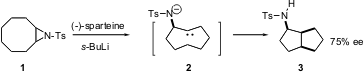
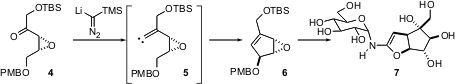The prochiral aziridine 1 is easily prepared from cyclooctene. Paul Müller of the University of Geneva has shown (Helv. Chim. Buy362522-50-7 Acta 2004, 87, 227. DOI: 10.1002/hlca.200490010)that metalation of 1 in the presence of the chiral amine sparteine leads to the bicyclic amine 3 in 75% ee, by way of intramolecular C-H insertion by the intermediate chiral carbene 2. PMID:23756629 The sparteine can be recovered and recycled. Formula of 4-Bromo-6-(trifluoromethyl)-1H-indole
As exemplified by the recent synthesis of (+)-trehazolin (7) by Susumu Ohira of Okayama University of Science (Tetrahedron Lett. 2004, 45, 7133.DOI: 10.1016/j.tetlet.2004.07.087), carbenes can also be conveniently generated by exposure of ketones such as 4 to lithiated TMS diazomethane. Intramolecular C-H insertion then proceeds with high diastereoselectivity, to give 6, a key intermediate on the way to 7.
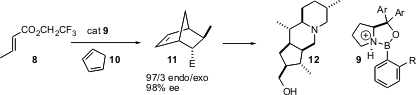
The central role of the Diels-Alder reaction as a workhorse forcarbocyclic construction has driven the development of effective chiral catalysts. The state of the art is represented by the catalyst 9, developed (J. Am. Chem. Soc. 2004, 126, 13708.DOI: 10.1021/ja046154m)by E.J. Corey of Harvard University. This catalyst is effective with a range of dienes and dienophiles, as illustrated by the combination of 8 and 10 to give the endo adduct 11 in high de and ee. The acid corresponding to 11 was a key starting material for the Aubé synthesis of 12, theDentrobatid alkaloid 251F.
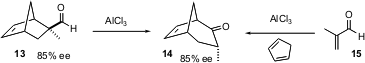
Cyclopentadiene Diels-Alder adducts such as 11 are highly strained. Huw M.L. Davies of the State University of New York, Buffalo, has established (J. Am. Chem. Soc. 2004, 126, 2692.DOI: 10.1021/ja039908q)an important strategic connection between Diels-Alder adducts such as 13 and the enantiomerically-enriched cyclohexane derivative 14. Oxidative cleavage of the alkene of 14 would lead to a cyclohexane derivative that would be difficult to access by other means. The same adduct 14 (albeit in racemic form) is also available directly by AlCl3-mediated addition of methacrolein 15 to cyclopentadiene.