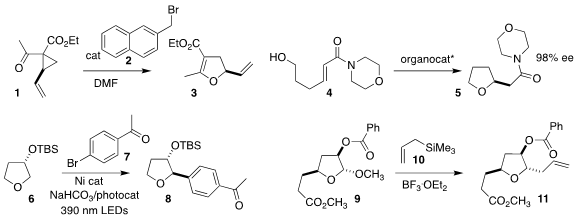
Guisheng Zhang and Zhiguo Zhang of Henan Normal University rearranged the
activated cyclopropane 1 with catalytic 2, leading to the
dihydrofuran
3
(J. Org. PMID:23800738 Chem. 2023, 88, 1003.
DOI: 10.1021/acs.joc.2c02415).
Trevor A. Hamlin of the Vrije Universiteit Amsterdam
and Darren J. Dixon of the University of Oxford achieved high enantioselectivity
in the bifunctional iminophosphorane-catalyzed cyclization of the unsaturated
amide 4 to the
tetrahydrofuran 5
(J. Am. D-Ala-D-Ala supplier 103128-76-3 Price Chem. Soc. 2023, 145, 12771.
DOI: 10.1021/jacs.3c03182).
Minyang Wang of Nanjing University and Wangqing Kong of Wuhan University showed that
depending on the choice of catalyst and protecting group, the coupling of
tetrahydrofuran 6 with the aryl bromide 7 could be directed to either the
2,3-product 8, or the regioisomeric 2,4-product
(J. Am. Chem. Soc. 2023, 145, 5231.
DOI: 10.1021/jacs.2c12481).
In support of a synthesis of eribulin, Feng Lin of WuXi AppTech and Wei
Li of China Pharmaceutical University showed that coupling of the
tetrahydrofuran 9 with the allyl silane 10 led to 11 with high
diastereoselectivity
(Org. Process Res. Dev. 2023, 27, 367.
DOI: 10.1021/acs.oprd.2c00370).
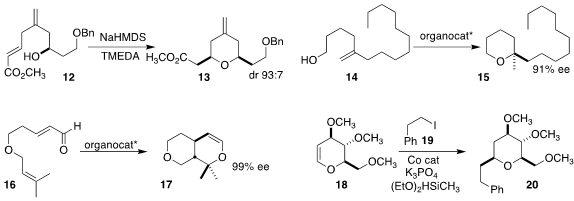
Robert Britton of Simon Fraser University reported a complementary approach to eribulin
(Nature Commun. 2023, 14, 1904.
DOI: 10.1038/s41467-023-37346-7).
Dzmitry G. Kananovich of the the Tallinn University of Technology and Iryna
V. Mineyeva of the Belarusian State University showed that depending on
conditions, cyclization of the unsaturated ester 12
could be directed toward either diastereomer of the
tetrahydropyran 13
(J. Org. Chem. 2023, 88, 355.
DOI: 10.1021/acs.joc.2c02382).
Nobuya Tsuji and Pavel Sidorov of Hokkaido University, Alexandre Varnek of
the University of Strasbourg and Benjamin List of the Max-Planck-Institut für
Kohlenforschung used a machine learning model to optimize an
imidophosphorimidate catalyst for the enantioselective cyclization of the
unsaturated alcohol 14 to the tetrahydropyran 15
(Angew. Chem. Int. Ed. 2023, 62, e202218659.
DOI: 10.1002/anie.202218659).
Yu Lan of Chongqing University and Lizhu Gao of Huaqiao
University used an oxaloborolidinium catalyst to cyclize the unsaturated
aldehyde 16 to the
bicyclic ether 17
(Nature Commun. 2023, 14, 3511.
DOI: 10.1038/s41467-023-39184-z).
Xi Lu and Yao Fuo of the University of Science and Technology of China and Qiang Lu of
Tsinghua University devised a protocol for coupling the dihydropyran 18 with the
iodide 19, leading to the tetrahydropyran 20
(Angew. Chem. Int. Ed. 2023, 62, e202218544.
DOI: 10.1002/anie.202218544).
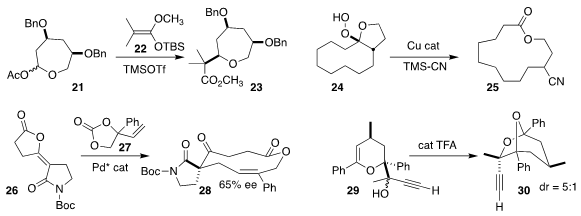
Jiang Wang and Hong Liu of the Shanghai Institute of Materia Medica
reported a parallel investigation
(Angew. Chem. Int. Ed. 2023, 62, e202300424.
DOI: 10.1002/anie.202300424).
K. A. Woerpel of New York University observed that the coupling of the
septanose acetate 21 with the ketene silyl acetal 22 led to the oxepane
23 with high diastereoselectivity
(Org. Lett. 2023, 25, 152.
DOI: 10.1021/acs.orglett.2c03963).
Building on the work of Irving J. Borowitz of Yeshiva University
(J. Org. Chem. 1968, 33, 2013.
DOI: 10.1021/jo01269a067)
and others, K. N. Houk of UCLA and Xin-Hua Duan and Li-Na Guo of Xi’an Jiaotong
University effected the cleavage and concommitant coupling with trimethylsilyl
cyanide of the hydroperoxide 24, leading to the
macrolactone 25
(Chem. Sci. 2023, 14, 5220,
DOI: 10.1039/D2SC06157K;
Org. Lett. 2023, 25, 4329,
DOI: 10.1021/acs.orglett.3c01427).
Guang-Yao Ran of Chongqing Medical University assembled the macrolactone 28 by
coupling the cyclic carbonate 27 with the enol lactone 26
(Org. Lett. 2023, 25, 2030.
DOI: 10.1021/acs.orglett.3c00374).
Boris M. Trofimov of the A. E. Favorsky Irkutsk Institute of Chemistry
cyclized the alkyne 29 to the bicyclic ketal 30
(Org. Biomol. Chem. 2023, 21, 3183.
DOI: 10.1039/D3OB00252G).
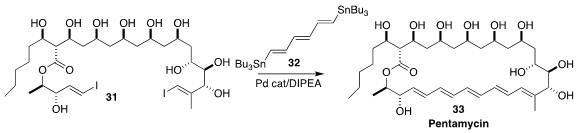
The polyene macrolide pentamycin (33), isolated from Streptomyces penticus, is
a clinically effective antibiotic. Dirk Menche of the Universität Bonn prepared
33 by coupling the unprotected polyol 31 with the triene 32
(J. Am. Chem. Soc. 2023, 145, 10974.
DOI: 10.1021/jacs.3c03011).