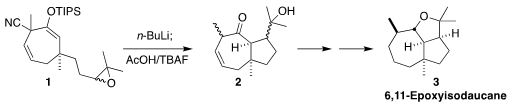
6,11-Epoxyisodaucane (3) was isolated from the liverwort Tritomaria
polita. In the course of the synthesis of 3, a key step of which was
the decyanative cyclization of the epoxide 1 to the ketone 2,
Keiji Tanino of Hokkaido University corrected the structure
(Org. Lett. Price of 1446022-58-7 2022, 24, 7939.
DOI: 10.1021/acs.orglett.2c03068). 1430219-73-0 web
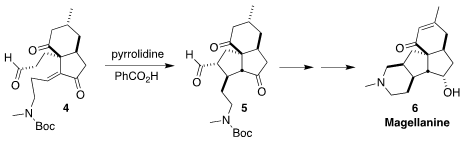
The tetracyclic alkaloid magellanine (6) was isolated from the club
moss Lycopodium magellanicum. Zhu-Jun Yao of Nanjing University assembled
the carbocyclic skeleton of 6 by the diastereoselective intramolecular
Michael cyclization of 4 to 5
(J. Org. Chem. PMID:24455443 2022, 87, 8685.
DOI: 10.1021/acs.joc.2c00871).
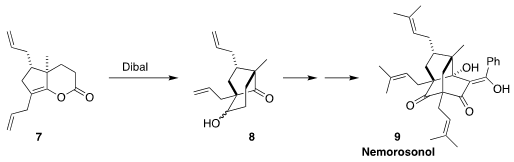
Nemorosonol (9), isolated from the Brazilian tree Clusia nemorosa,
shows antibiotic activity. En route to 9, Takayuki Ohyoshi and Hideo
Kigoshi of the University of Tsukuba reduced the enol lactone 7 with
Dibal, leading to the aldol product 8
(Org. Lett. 2022, 24, 4635.
DOI: 10.1021/acs.orglett.2c01745).
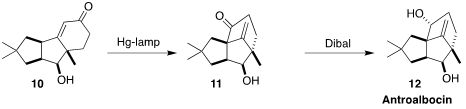
Antroalbocin (12), isolated from the fungus Antrodiella
albocinnamomea, also showed antibiotic activity. Markus Kalesse of the
Gottfried Wilhelm Leibniz University Hannover irradiated the enone 10 to
give first the β,γ-unsaturated ketone, that underwent 1,3-acyl migration to
11. Diastereoselective reduction completed the synthesis of 12
(Org. Lett. 2022, 24, 5812.
DOI: 10.1021/acs.orglett.2c02347).
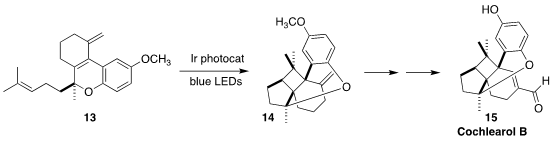
Cochlearol B (15) was isolated from the fruiting body of the fungus Ganoderma
cochlear, used in traditional Chinese medicine. Corinna S. Schindler of the
University of Michigan showed that the Ir photocatalyst was essential for the
efficient cyclization of 13 to 14
(Angew. Chem. Int. Ed. 2022, 61, e202201213.
DOI: 10.1002/anie.202201213).
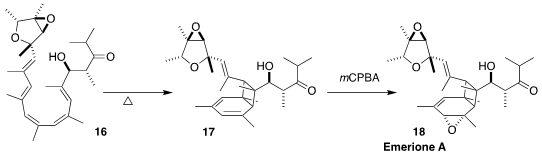
Emerione A (18), isolated from the fungus Emericella nidulans, inhibits NO
production in lipopolysaccharide-induced RAW264.7 cells. Aubry K. Miller of the
German Cancer Research Center prepared 17 by the gentle warming of the pentaene
16. Selective epoxidation completed the synthesis of 18
(Angew. Chem. Int. Ed. 2022, 61, e202205878.
DOI: 10.1002/anie.202205878).