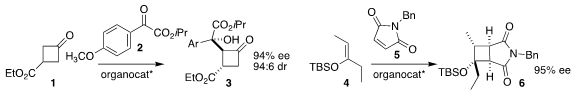Xiaoxia Ye of Wenzhou Medical University and Jun Jiang of Wenzhou University
used the barium salt of proline to direct the
aldol reaction of the prochiral
cyclobutanone 1 with the α-ketoester 2, leading to the diester 3
(Synlett 2022, 33, 1619.
DOI: 10.1055/s-0040-1719931).
Rong-Jie Chein of the Academia Sinica used a oxathiaborolium catalyst
to mediate the [2+2] cycloaddition of the silyl enol ether 4 with the maleimide
5, to give the
cyclobutane 6
(Org. Biomol. Chem. PMID:23912708 2022, 20, 8405.
DOI: 10.1039/D2OB01779B).
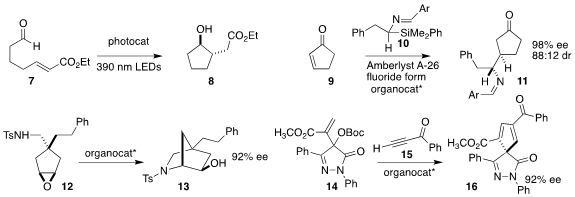
David A. Nicewicz of the University of North Carolina employed an acridinium
photocatalyst to cyclize the aldehyde 7 to the
cyclopentanol 8
(J. Am. Chem. Soc. 2022, 144, 11888.
DOI: 10.1021/jacs.2c04822).
Jisheng Luo and Li Deng of Westlake University used a
bisphosphine catalyst to set the absolute configuration of the
conjugate
addition of the imine 10 to
cyclopentenone 9, leading to 11
(J. Am. 213125-87-2 web Chem. Soc. 2022, 144, 23264.
DOI: 10.1021/jacs.2c10777).
Aijun Lin and Hequan Yao of China Pharmaceutical University
showed that a BINOL-derived phosphoric acid was effective for cyclizing the
prochiral epoxide 12 to the bicyclic amine 13
(Org. Lett. 1255099-26-3 site 2022, 24, 8791.
DOI: 10.1021/acs.orglett.2c03529).
Baomin Wang of the Dalian University of Technology used a Cinchona-derived
catalyst to construct the cyclopentadiene 16 by the combination of the carbonate
14 with the alkynyl ketone 15
(Chem. Commun. 2022, 58, 9504.
DOI: 10.1039/D2CC02963D).
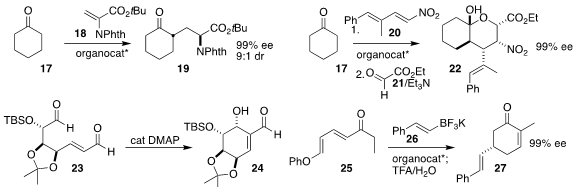
Giovanni Piersanti of the University of Urbino Carlo Bo used a
diamine-derived thiourea to catalyze the addition of
cyclohexanone 17 to the
dehydroalanine 18, leading to the amine 19 in high ee
(Chem. Eur. J. 2022, 28, e202201994.
DOI: 10.1002/chem.202201994).
María de Gracia Retamosa of the Universidad de Alicante and Fernando P. Cossio of the University of the Basque Country
showed that a γ-dipeptide effectively catalyzed the addition of cyclohexanone 17 to the nitrodiene 20 to
give, after the addition of ethyl glyoxylate 21, the ester 22
(J. Org. Chem. 2022, 87, 14819.
DOI: 10.1021/acs.joc.2c01230).
Xiaochuan Chen of Sichuan University used DMAP to cyclize the
dialdehyde 23 to the
cyclohexanol 24
(J. Org. Chem. 2022, 87, 14636.
DOI: 10.1021/acs.joc.2c01992).
Jeremy A. May of the University of Houston employed a BINOL derivative to mediate the
enantioselective conjugate addition of the trifluoroborate 26 to the dienone 25,
leading after intramolecular
aldol condensation to the 5-substituted
cyclohexenone 27
(Org. Lett. 2022, 24, 5334.
DOI: 10.1021/acs.orglett.2c01976).
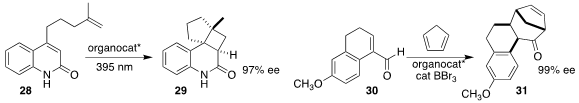
Anthony P. Green of the University of Manchester designed a photocatalytic
enzyme that directed the cyclization of the diene 28 to the
cyclobutane 29
(Nature 2022, 611, 709.
DOI: 10.1038/s41586-022-05335-3).
Manabu Hatano of Kobe Pharmaceutical University and
Kazuaki Ishihara of Nagoya University used a BINOL-derived phosphoric acid
enhanced by BBr3 to mediate the
Diels-Alder addition of the unsaturated aldehyde
30 to cyclopentadiene, leading, after acid-mediated ring expansion, to the
tetracyclic ketone 31
(Org. Lett. 2022, 24, 6483.
DOI: 10.1021/acs.orglett.2c02747).
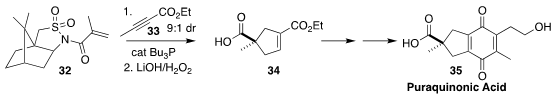
Puraquinonic acid 35, produced by cultures of the fungus Mycena pura, induces
differentiation of HL-60 cells (human promyelocytic leukemia)
and may therefore be a lead compound in the design of drugs to treat leukemia.
En route to 35, Ken-ichi Takao of Keio University added the alkyne
33 to the Oppolzer acryloylsultam 32, leading to the monoester
34 in high ee
(J. Org. Chem. 2022, 87, 8788.
DOI: 10.1021/acs.joc.2c00753).