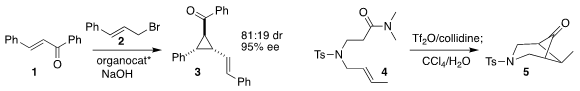
Rong-Jie Chein of the Academia Sinica, Taipei used the known
(S)-(thiolan-2-yl) diphenylmethanol benzyl ether to catalyze the
Johnson-Corey-Chaykovsky cyclopropanation of the enone 1 with the allylic bromide 2 to give the
cyclopropane 3
(J. Org. 2-Iodo-4-methoxybenzonitrile Order Chem. 2023, 88, 559.
DOI: 10.1021/acs.joc.2c02573).
Zhi-Xiang Yu of Peking University optimized the cyclization of the amide 4 to the
cyclobutanone 5
(J. Am. Chem. Soc. 2023, 145, 9634.
DOI: 10.1021/jacs.3c00685).
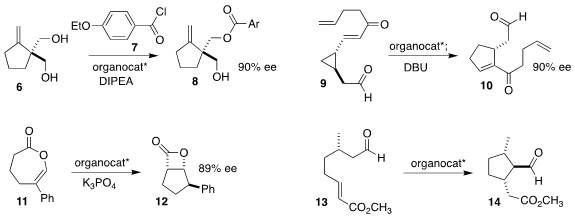
Wen-Hua Zheng of Nanjing University used a chiral oxaboraanthracene to
mediate the enantioselective acylation of the prochiral diol 6 with the acid
chloride 7, leading to the ester 8
(J. Am. Chem. Soc. 2023, 145, 8338.
DOI: 10.1021/jacs.3c02331).
Efraim Reyes and Jose L. Vicario of the University of the Basque Country and Pedro
Merino of the Universidad de Zaragoza used a diphenylprolinol sily ether to
rearrange the alkenyl cyclopropane 9 to the
cyclopentene 10
(Angew. Chem. PMID:27217159 Int. Ed. 2023, 62, e202302416.
DOI: 10.1002/anie.202302416).
Song Ye of the Institute of Chemistry of the Chinese
Academy of Sciences prepared the
cyclopentane
12 by rearranging the prochiral
lactone 11 with a chiral N-heterocyclic carbene
(Org. Chem. Formula of Tetrahydro-2H-pyran-4-carbaldehyde Front. 2023, 10, 799.
DOI: 10.1039/D2QO01721K).
Bor-Cherng Hong of the National Chung Cheng University and Su-Ying Chien
of the National Taiwan University showed that a diphenylprolinol sily ether
cyclized the aldehyde 13 to the ester 14
(Org. Biomol. Chem. 2023, 21, 4200.
DOI: 10.1039/D3OB00439B).
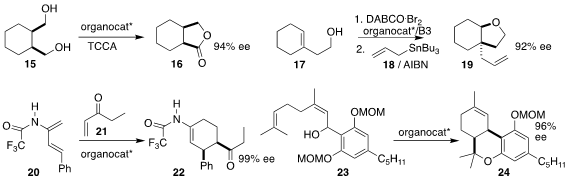
Matthew S. Sigman of the University of Utah, Scott J. Miller of Yale
University and Song Lin of Cornell University designed a chiral aminoxyl radical
that directed the trichloroisocyanuric acid oxidation of the diol 15 to the
lactone 16
(Science 2023, 380, 706.
DOI: 10.1126/science.adf6177).
Hidetoshi Tokuyama of Tohoku University
cyclized the homoallylic alcohol 17 with DABCO bromine complex and a
binaphthol-based chiral phosphoric acid to give an intermediate that they then
coupled with the allyl stannane 18, leading to the
cyclohexane
19 containing a stereodefined quaternary center
(Tetrahedron Lett. 2022, 101, 153906.
DOI: 10.1016/j.tetlet.2022.153906).
Jieping Zhu of the Ecole Polytechnique Fédérale de Lausanne also used a binaphthol-based
chiral phosphoric acid to direct the course of the
Diels-Alder cycloaddition of
the enone 21 to the diene 20, leading to 22
(Angew. Chem. Int. Ed. 2023, 62, e202214925.
DOI: 10.1002/anie.202214925).
A chiral imidodiphosphorimidate successfully catalyzed the
cyclization of the diene 23 to the
cyclohexene
24, as reported by Christoph
Schneider of the Universität Leipzig
(Angew. Chem. Int. Ed. 2023, 62, e202302475.
DOI: 10.1002/anie.202302475).
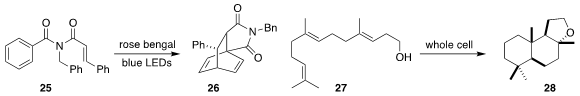
Furong Wang and Biaolin Yin of the South China University of Technology and
Xiaohui Cao of Guangdong Pharmaceutical University showed that catalytic rose
bengal directed the photocyclization of the imide 25 exclusively to the [4+2]
product 26
(Adv. Synth. Catal. 2023, 365, 43.
DOI: 10.1002/adsc.202201110).
Sílvia Osuna of the Universitat
de Girona and Bernhard Hauer of the University of Stuttgart redesigned
squalene-hopene cyclase, leading to a whole cell system that efficiently
converted homofarnesol 27 to the commercially-important ambroxide 28
(Angew. Chem. Int. Ed. 2023, 62, e202301607.
DOI: 10.1002/anie.202301607).
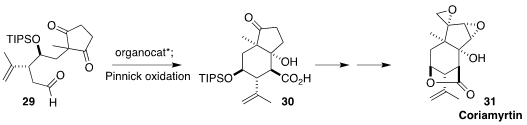
Coriamyrtin (31) is a toxin found in high concentration in the berries of
Coriaria myrtifolia, one of the most neurotoxic of the plants of the western
Mediterranean. Kazutada Ikeuchi, now at Nagoya City University and Keiji Tanino
of Hokkaido University showed that a benzimidazole-pyrrolidine cyclized the
symmetrical diketone 29 with high diastereoselectivity, leading after oxidation
to the carboxylic acid 30, that was carried on to 31
(Org. Lett. 2023, 25, 2751.
DOI: 10.1021/acs.orglett.3c00249).