Acyclic stereoarrays are important both in themselves and as precursors to enantiomerically-defined ring systems. Although the aldol condensation has for many years been a workhorse for acyclic stereoselection, there are still new things that can be done.
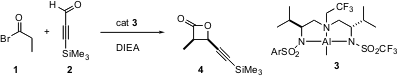
In a pair of papers last year, Scott Nelson of the University of Pittsburgh expanded the range of the ketene “aldol”. PMID:36628218 1025796-31-9 manufacturer In the first paper (J. Am. Chem. Soc. 2004, 126, 14. DOI: 10.1021/ja0391208), he employed a chiral Al-based catalyst 3. 1196157-42-2 uses This catalyst mediated additions such as propionyl bromide 1 to 2 to give 4 in 98:2 syn/anti ratio and 95% ee.
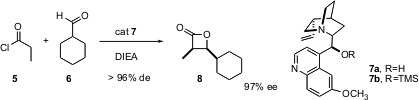
In a follow-up paper (J. Am. Chem. Soc. 2004, 126, 5352. DOI: 10.1021/ja0492900), Professor Nelson used the commercially-available base quinaldine 7a (R=H) or its TMS ether 7b (R=TMS). Catalysts 7a and 7b are both efficient and give > 95% ee, but lead to opposite absolute configurations of the products. As with catalyst 3, the syn aldol product predominates, but now branched aldehydes such as 6 also participate efficiently in the reaction. This is another example of enantioselective catalysis by a small organic molecule.
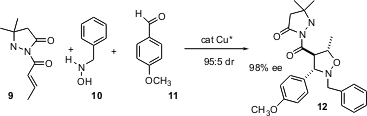
Mukund Sibi of North Dakota State University has developed (J. Am. Chem. Soc. 2004, 126, 718. DOI: 10.1021/ja039087p)a powerful three-component coupling, combining an α,β-unsaturated amide 9, a hydroxylamine 10, and an aldehyde11. The hydroxylamine condenses with the aldehyde to give the nitrone, which then adds in a dipolar sense to the unsaturated ester. The reaction proceeds with high diastereocontrol, and the absolute configuration is set by the chiral Cu catalyst. As the amide 9 can be prepared by condensation of a phosphonacetate with another aldehyde, the product 12 can be seen as the product of a four-component coupling, chirally-controlled aldol addition and Mannich condensation on a starting acetamide.