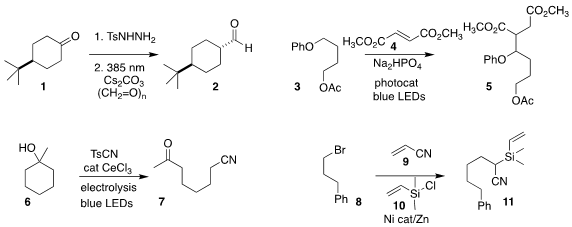
Burkhard König of University Regensburg observed significant
diastereoselectivity in the conversion of the ketone 1 to the aldehyde 2
(Angew. Chem. Int. PMID:30125989 Ed. 2022, 61, e202211578.
DOI: 10.1002/anie.202211578).
John A. Murphy of the University of
Strathclyde used a photocatalyst to activate the aryl alkyl ether 3 and add it
in a conjugate sense to dimethyl fumarate 4, leading to the diester 5
(Chem. Sci. 2022, 13, 12921.
DOI: 10.1039/D2SC04463C).
Zhiliang Huang and Aiwen Lei of Wuhan University effected the
oxidative cleavage of the tertiary alcohol 6 to the keto nitrile 7
(J. Am. Chem. 1041026-70-3 Order Soc. Price of (R)-N-Fmoc-2-(7-octenyl)Alanine 2022, 144, 13895.
DOI: 10.1021/jacs.2c05520).
Xuan Zhang of the Nanjing University of Science
Information and Technology added the bromide 8 to acrylonitrile 9, trapping the
intermediate with the chlorosilane 10 to give the α-silyl nitrile 11
(Nature Commun. 2022, 13, 7093.
DOI: 10.1038/s41467-022-34901-6).
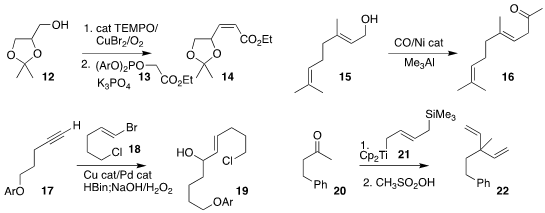
Kaori Ando of Gifu University effected the one-pot oxidation of the alcohol
12 and homologation of the intermediate aldehyde with the reagent 13, leading to
the Z-unsaturated ester
14
(J. Org. Chem. 2022, 87, 9723.
DOI: 10.1021/acs.joc.2c00763).
Yifeng Chen of the East China University of Science and Technology
carbonylated the allylic alcohol
15 to give the ketone 16
(Angew. Chem. Int. Ed. 2022, 61, e202210484.
DOI: 10.1002/anie.202210484).
Gojko Lalic of the University of Washington assembled the allylic alcohol 19 by
coupling the alkyne 17 with the alkenyl bromide 18
(Angew. Chem. Int. Ed. 2022, 61, e202206462.
DOI: 10.1002/anie.202206462).
By adding the reagent 21 to the ketone 20, Keiji Tanino of
Hokkaido University converted it into the prochiral 1,4-diene 22
(Org. Lett. 2022, 24, 5040.
DOI: 10.1021/acs.orglett.2c01799).
Gerald B. Hammond of the University of Louisville and Bo Xu of Donghua
University assembled the bromo alkyne 25 by the selective
coupling of the alkynyl trifluoroborate
24 with the bromo triflate 23
(Org. Lett. 2022, 24, 6298.
DOI: 10.1021/acs.orglett.2c02507).
Ohyun Kwon of UCLA effected the oxidative coupling of the alkene 26 with
the alkynyl sulfone 27, leading to the ester alkyne 28
(J. Am. Chem. Soc. 2022, 144, 14828.
DOI: 10.1021/jacs.2c05980).
Chang Guo of the University of Science and Technology of China used the reagent
30 to convert the racemic propargylic alcohol 29 to the
allene 31 in high ee
(J. Am. Chem. Soc. 2022, 144, 21022.
DOI: 10.1021/jacs.2c10863).
Liang Yin of SIOC constructed the
allene 34 in high ee by coupling the amide 32 with the imine 33
(ACS Catal. 2022, 12, 9181.
DOI: 10.1021/acscatal.2c01399).
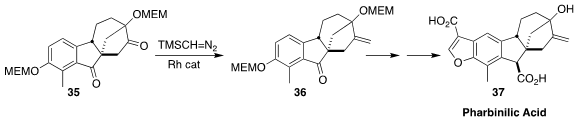
Pharbinilic acid (37), isolated from the morning glory Pharbitis nil, has been
converted into derivatives having antiproliferative activity. In the course of
the assembly of a known intermediate in the synthesis of 37, Huilin Li and
Xuegong She of Lanzhou University used the Lebel protocol to prepare 36 by the
selective methylenation of the diketone 35
(Org. Lett. 2022, 24, 6402.
DOI: 10.1021/acs.orglett.2c02422).